Figure 3. Azetidine acrylamide-based heterobifunctional compounds stereoselectively engage DCAF1.
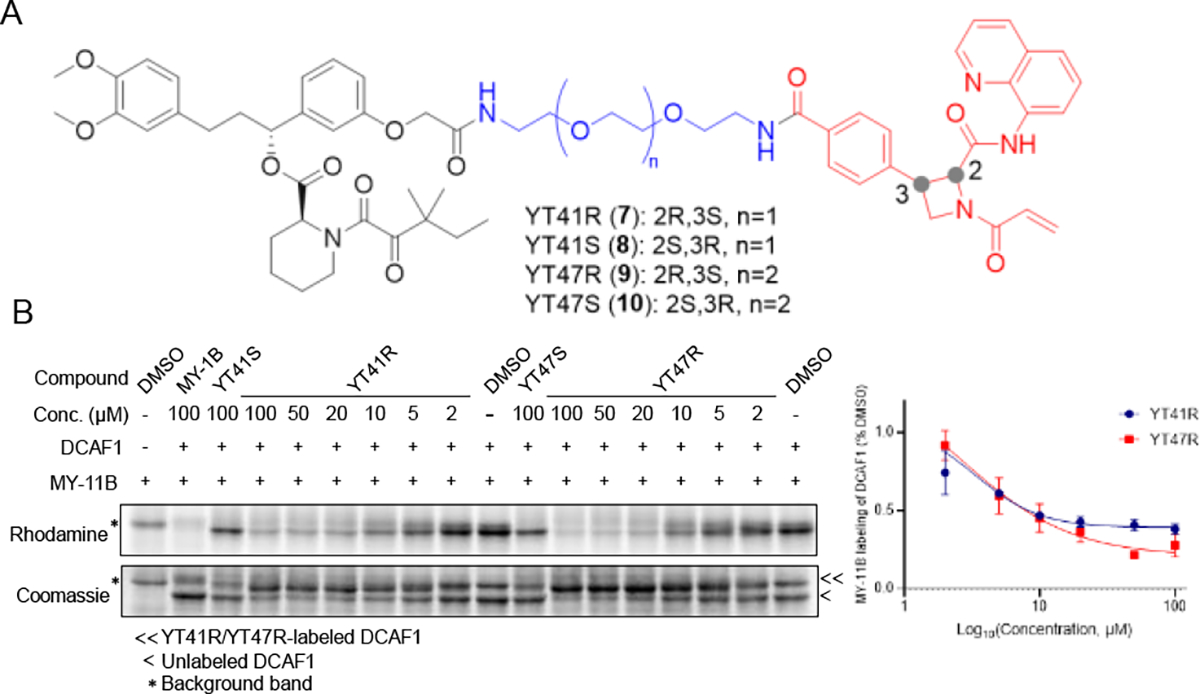
(A) Structures of candidate electrophilic PROTACs YT41R (7), YT41S (8), YT47R (9), and YT47S (10). (B) Gel-ABPP showing the concentration-dependent effects of YT41R and YT47R on MY-11B reactivity with DCAF1-WT protein (0.06 μg/μL of DCAF1 protein per sample) doped into HEK293T cell proteome (1 μg/μL). Also shown are the effects of YT41S and YT47S tested at a single concentration (100 μM). Samples were treated with heterobifunctional compounds for 2 h, followed by MY-11B (25 μM, 1 h) and analyzed as described in Figure 2D. Left, representative gel-ABPP results. Lower image is Coomassie blue-stained gel corresponding to ABPP gel shown in upper image. Right, quantification of data presented as mean values ± SEM from two independent experiments.
