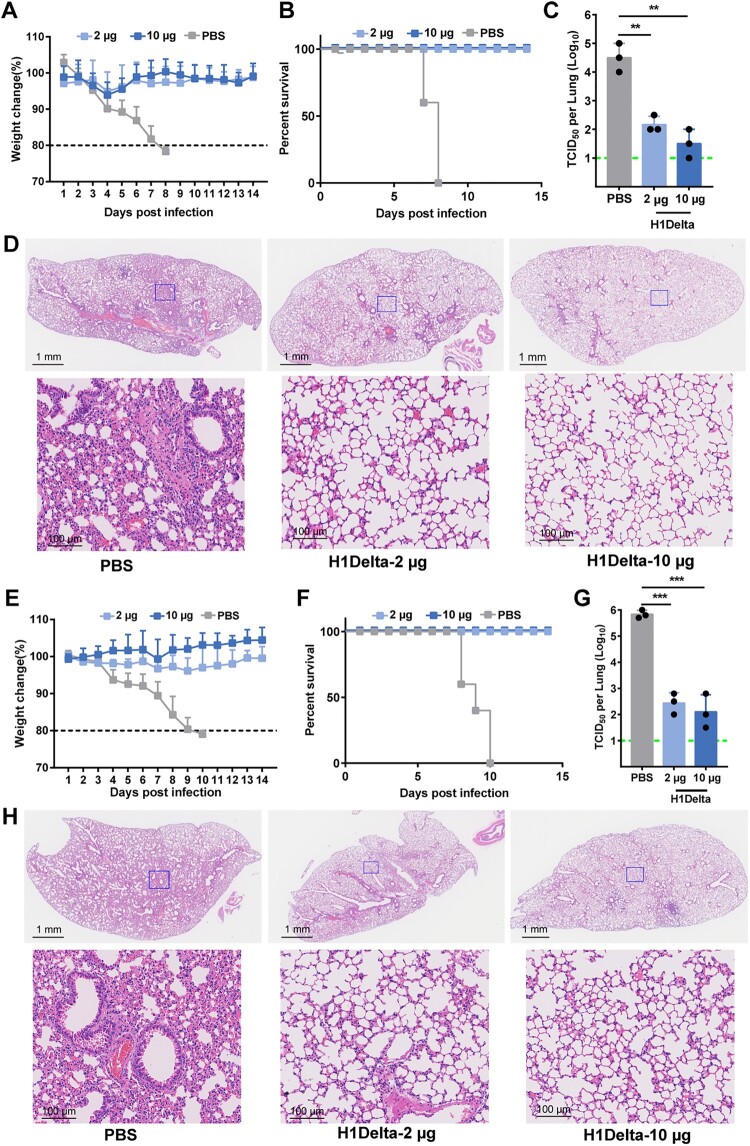
Figure 3.
Protection efficacy of the H1Delta protein vaccine to homologous H1N1 and heterologous H5N8. (A-D) Groups of 6-to-8-week-old female BALB/c mice (n = 8) were vaccinated with three immunizations of 2 or 10 μg immunogen with an adjuvant of AddaVax in 3-week intervals. PBS with the adjuvant was given as a control. Serum samples were collected 35 and 56 days after initial immunization. Mice were intranasally challenged with 20 LD50 of A/Brisbane/02/2018 (H1N1) virus. Lung tissues were harvested and split for virus titer detection (n = 3) and pathological examination (n = 3), respectively. Vaccine efficacy was assessed by measuring (A) morbidity (weight loss), (B) mortality (survival), (C) lung viral titers on day 3 post challenge, and (D) histological pathology analyses. (E-H) Groups of 6-to-8-week-old female BALB/c mice (n = 8) were vaccinated with three immunizations of 2 or 10 μg immunogen with an adjuvant of AddaVax in 3-week intervals. PBS with the adjuvant was used as a control. Serum samples were collected 35 and 56 days after initial immunization. Mice were intranasally challenged with 10 LD50 of reassortment A/Astrakhan/3212/2020(H5N8) virus. Lung tissues were harvested and split for virus titer detection (n = 3) and pathological examination (n = 3), respectively. Vaccine efficacy was assessed by measuring (E) morbidity (weight loss), (F) mortality (survival), (G) lung viral titers on day 3 post challenge, and (H) histological pathology analyses. Differences were compared using two-tail unpaired t test (**p < 0.01 and ***p < 0.001).