Fig. 4. The evolution, cellular and ecological roles for the DMSP lyase enzyme in phytoplankton.
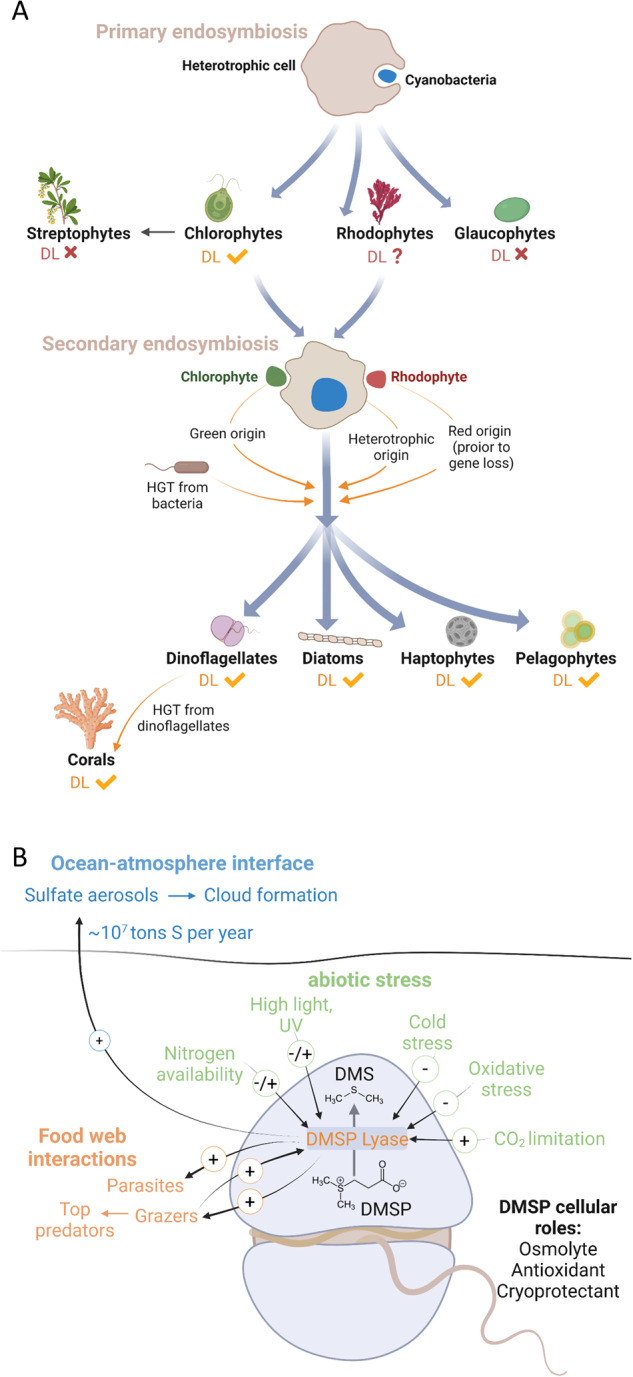
A Possible acquisition pathways for the DL enzyme in haptophytes, dinoflagellates, diatoms and pelagophytes. Primary endosymbiosis gave rise to chlorophytes, including species which possess DLHs, and to rhodophytes and glaucophytes, which their DLH are either missing or remained to be identified. Secondary endosymbiosis gave rise to haptophytes, dinoflagellates, diatoms and pelagophytes, which all have representative species encoding for DLHs. The DL evolutionary origin in those phytoplankton groups may be attributed to the heterotrophic host that engulfed a rhodophyte or chlorophyte, to a rhodophyte (considering unidentified DL, or that DL gene loss in red genomes occurred after secondary endosymbiosis), or to chlorophytes [40] or bacteria via horizontal gene transfer (HGT) [12]. Corals DLHs were probably horizontally transferred from their dinoflagellate symbionts [19, 20]. B Proposed cellular and ecological roles for the DL enzyme under stress conditions. DL enzymatic activity or transcription was linked (up- or down-regulated, depending on tested species) to nitrogen levels (this study, and ref. [29, 46]); high light and ultraviolet (UV) radiation (this study, and ref. [42, 44, 45]; cold stress [22]; carbon dioxide [42], and oxidative stress [1]. During microbial interactions, DMS acts as an activation cue for parasites [7] and plays a pro-grazing role for protist grazers [6] and copepods [51]. DMS is also accumulated in the water during grazing, serving as chemoattractant for diverse top predators [66]. In addition to the mentioned cellular and ecological roles, DMS plays a climatic role; the ocean-atmosphere DMS flux is estimated as 10 million tons S per year [2], and atmospheric DMS is rapidly oxidized to form sulfate aerosols, enhancing cloud formation and increases the albedo of earth.
