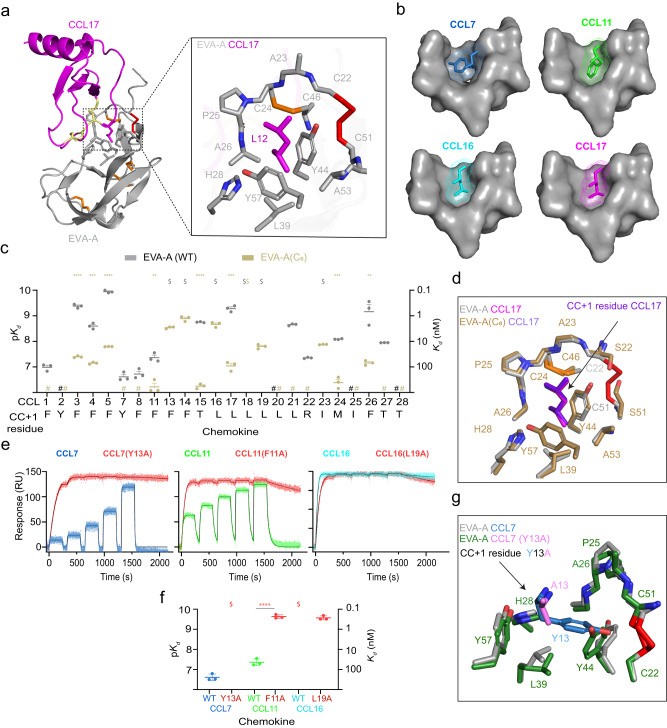
Fig. 3. The fifth disulfide defines a critical binding pocket for chemokine target selectivity (CC + 1 residue binding).
a Cartoon representation of EVA-A CCL7, with expanded view showing the EVA-A residues (grey, additional disulfide: red) of the hydrophobic pocket holding the CC + 1 residue (magenta) of CCL17. b Surface representations of the EVA-A binding pocket (grey) fitting the CC + 1 residue of four different chemokines; CCL7 (sky blue), CCL11 (green), CCL16 (cyan) and CCL17 (magenta). c Chemokine affinities (Kd) of EVA-A (grey) and EVA-A(C8) (sand). Data are presented as mean ± SEM from three independent experiments. $, Kd < 0.1 nM; #, no measurable binding at 500 nM chemokine concentration; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, versus wild type EVA-A (two-tailed t test with Holm- Šídák correction for multiple comparisons). d Overlay of the binding pocket residues of EVA-A (grey) bound to the CC + 1 residue of CCL17 (magenta) and EVA-A(C8) (sand) bound to the CC + 1 residue of CCL17 (purple). e Representative SPR sensorgrams for binding of EVA-A to wild type chemokines (CCL7, sky blue; CCL11, green; and CCL16, cyan) and CC + 1 residue-mutated versions of each chemokine (red) measured using single-cycle kinetics (5 chemokine injections at consecutive concentrations of 31.25, 63.5, 125, 250 and 500 nM). f EVA-A affinities (Kd) for wild type and CC + 1 residue-mutated chemokines (coloured as in e). Data are presented as mean ± SEM from three independent experiments. $, Kd < 0.1 nM; ****p < 0.0001, versus wild type chemokine (two-tailed t test). g Overlay of the binding pocket residues of EVA-A (grey) bound to CCL7 (sky blue) and EVA-A (forest green) bound to CCL7(Y13A) (violet).