Abstract
Background
Colistin (CST) is a last-line drug for multidrug-resistant Gram-negative bacterial infections. CST-heteroresistant Enterobacter cloacae complex (ECC) has been isolated. However, integrated analysis of epidemiology and resistance mechanisms based on the complete ECC species identification has not been performed.
Methods
Clinical isolates identified as “E. cloacae complex” by MALDI-TOF MS Biotyper Compass in a university hospital in Japan were analyzed. Minimum inhibitory concentrations of CST were determined by the broth microdilution method. The population analysis profiling (PAP) was performed for detecting the heteroresistant phenotype. The heat shock protein 60 (hsp60) cluster was determined from its partial nucleotide sequence. From the data of whole-genome sequencing, average nucleotide identity (ANI) for determining ECC species, multilocus sequence type, core genome single-nucleotide-polymorphism-based phylogenetic analysis were performed. phoPQ-, eptA-, and arnT-deleted mutants were established to evaluate the mechanism underlying colistin heteroresistance. The arnT mRNA expression levels were determined by reverse transcription quantitative PCR.
Results
Thirty-eight CST-resistant isolates, all of which exhibited the heteroresistant phenotype by PAP, were found from 138 ECC clinical isolates (27.5%). The prevalence of CST-resistant isolates did not significantly differ among the origin of specimens (29.0%, 27.8%, and 20.2% for respiratory, urine, and blood specimens, respectively). hsp60 clusters, core genome phylogeny, and ANI revealed that the CST-heteroresistant isolates were found in all or most of Enterobacter roggenkampii (hsp60 cluster IV), Enterobacter kobei (cluster II), Enterobacter chuandaensis (clusters III and IX), and Enterobacter cloacae subspecies (clusters XI and XII). No heteroresistant isolates were found in Enterobacter hormaechei subspecies (clusters VIII, VI, and III) and Enterobacter ludwigii (cluster V). CST-induced mRNA upregulation of arnT, which encodes 4-amino-4-deoxy-l-arabinose transferase, was observed in the CST-heteroresistant isolates, and it is mediated by phoPQ pathway. Isolates possessing mcr-9 and mcr-10 (3.6% and 5.6% of total ECC isolates, respectively) exhibited similar CST susceptibility and PAP compared with mcr-negative isolates.
Conclusions
Significant prevalence (approximately 28%) of CST heteroresistance is observed in ECC clinical isolates, and they are accumulated in specific species and lineages. Heteroresistance is occurred by upregulation of arnT mRNA induced by CST. Acquisition of mcr genes contributes less to CST resistance in ECC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12941-023-00610-1.
Keywords: Enterobacter cloacae complex, Colistin, Heteroresistance, arnT, PhoPQ, Average nucleotide identity (ANI)
Background
Enterobacter cloacae complex (ECC) and Enterobacter aerogenes (currently as Klebsiella aerogenes) represent the most frequently isolated Enterobacter spp. in respiratory, urinary tract, and bloodstream infections [1, 2]. According to recent literatures, following species, Enterobacter asburiae, Enterobacter cancerogenus, Enterobacter cloacae, Enterobacter hormaechei, Enterobacter kobei, Enterobacter ludwigii, Enterobacter mori, Enterobacter nimipressuralis, Enterobacter roggenkampii, and Enterobacter soli are assigned to ECC [3–5]. However, the classification has been expanding due to the application of whole-genome sequencing (WGS) to bacterial species identification. The clinical relevancy and antimicrobial resistance of individual ECC species are unclear because routine bacterial identification procedures, such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and conventional biochemical testing with automated systems, used in clinical laboratories have not stay in step with classification of ECC into individual species [3]. Whereas heat shock protein 60 gene (hsp60) clustering have been used to classify ECC clinical isolates [6], DNA–DNA hybridization and average nucleotide identity (ANI) are the most reliable bacterial identifications at present [7].
Significant numbers of multidrug-resistant ECC have emerged [8, 9]. Thus, the choice of antimicrobials in the treatment of their infections appears to be becoming difficult. Colistin (CST) is a polycationic antimicrobial peptide that is effective and used as a last-line drug for multidrug-resistant Gram-negative bacteria infections, including ECC; however, CST-resistant ECC has emerged in clinical settings worldwide [6, 10, 11].
CST resistance is acquired by upregulation of the intrinsic lipid A-modifying enzyme gene, eptA (also known as pmrC) or arnT (present in the arn operon, arnBCADTEF), which encode the lipid A-modifying enzymes, phosphoethanolamine (pEtN) transferase and 4-amino-4-deoxy-l-arabinose (l-Ara4N) transferase, respectively [12]. Modification of lipid A decreases the negative charge of the outer membrane to repulse the positive charge of CST, leading to CST resistance [13]. Activation of two-component regulatory systems, PhoPQ and PmrAB, upregulates expression of the eptA and arn operon, and MgrB negatively regulates the two component system [12]. The mutations of phoPQ, mgrB, and/or pmrAB mediate CST resistance [14]. In addition, the worldwide spread of plasmid-mediated CST resistance genes, mcr-1 to mcr-10 has also been reported [15].
The CST resistance mechanism of ECC reported in the E. cloacae subsp. cloacae ATCC 13047 strain, which displays the CST-heteroresistant phenotype, involves the PhoPQ two-component system and arn operon [16]. CST heteroresistance refers to a bacterial clone containing both CST-susceptible and -resistant subpopulations, and causes therapeutic failure [17]. Thus, it necessitates assessment of CST-resistant subpopulations during clinical diagnosis of ECC infections.
In daily clinical laboratory investigations of antimicrobial susceptibility testing, the skip-well phenomenon with the broth microdilution method and the appearance of colonies in the inhibitory zone with the disc diffusion test and E-test are indications of the heteroresistant phenotype [11, 18]. Population analysis profiling (PAP) is the most reliable method to identify CST heteroresistance [19]. Unfortunately, identifications for ECC species and/or the CST heteroresistance in previous studies were inadequate [6, 10, 11, 16, 17].
This study aimed to elucidate the relationship between the genetic features and CST resistance mechanism by using the integrated analysis with molecular epidemiology [hsp60 clustering, multilocus sequence typing (MLST), core genome analysis, and species identification by ANI] and the CST-heteroresistance [evaluations of skip-well phenomenon, PAP test, bacterial growth, and contributions of phoPQ, mgrB, pmrAB, eptA, and arnT] to comprehensively understand CST resistance in ECC clinical isolates.
Methods
Bacterial isolates
ECC isolates (n = 138) were derived from clinical specimens of different patients at Sapporo Medical University Hospital (Sapporo, Japan) from May 2017 to May 2018. These isolates were identified as “E. cloacae complex” by MALDI-TOF MS Biotyper Compass (software version 4.1.80.24), bacterial library version 8 (Bruker, Billerica, MA) in clinical laboratory testing. We also collected K. aerogenes (formerly, E. aerogenes) isolates (n = 30). These clinical specimens were respiratory (n = 78, 46.4%), urine (n = 22, 13.1%), blood (n = 12, 7.2%), and others (n = 56, 33.3%).
Antimicrobial susceptibility testing, monitoring of bacterial growth, and detection of the skip-well phenomenon
CST, ampicillin, meropenem, gentamicin, and amikacin were purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan). Cefotaxime and ciprofloxacin were purchased from Tokyo Chemical Industry (Tokyo, Japan). Minimum inhibitory concentration (MIC)s were determined by the broth microdilution method using cation-adjusted Mueller–Hinton II broth (MHII broth; Becton, Dickinson and Co., Franklin Lakes, NJ) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [20]. Isolates with a CST MIC of ≥ 4 mg/L were defined as CST-resistant according to CLSI M100-S32.
Bacterial growth was measured by assessing turbidity (OD600) after cultivation for 20 h under the same condition as used for the susceptibility test in the presence of CST (0–128 mg/L). Turbidity was measured using an Infinite M200 PRO instrument (Tecan, Kawasaki, Japan). The skip-well phenomenon was defined as no growth (OD600 < 0.1) at a certain CST concentration(s) and growth (OD600 > 0.5) at higher CST concentrations. Data are expressed as the means ± standard deviations from independent triplicate experiments.
PAP
Heteroresistance was determined by PAP as previously described [19]. Briefly, ECC clinical isolates were inoculated into 10 mL of Trypticase Soy Broth (TSB) (Beckton, Dickinson and Co.) and cultured overnight at 37 ℃. The bacterial cell culture (approximately 1 × 109 CFU/mL) was centrifuged and resuspended in 1 mL of sterile 0.85% NaCl. One hundred microliters of tenfold serial dilutions (from 1 × 1010 to 1 × 103 CFU/mL) of the suspension were spread onto cation-adjusted Mueller–Hinton II agar (MHII agar; Becton, Dickinson and Co.) plates containing 0, 0.125, 1, or 8 mg/L CST and cultured for 20 h at 37 ℃. The occurrence frequencies of the CST-resistant subpopulations were calculated by dividing the numbers of CFU on CST-containing MHII agar plates by those on plain MHII agar plates. CST heteroresistance was determined using a previously published method based on the following criteria [19, 21]: (1) bacterial growth was confirmed in the presence of > 2 mg/L CST by the broth microdilution method, and (2) the CST concentration in the presence of which the CST-resistant subpopulations grew (within a frequency range from 1 × 10–7 to 1 × 10–1) were at least eightfold higher (≥ 1 mg/L CST) than the highest CST concentration that did not affect the growth of CST-susceptible subpopulations (0.125 mg/L CST). Data are expressed as the means ± standard deviations from three independent experiments.
hsp60 clustering
The 273 nucleotides of hsp60 fragment was amplified by PCR with specific primers as described previously [22]. The nucleotide sequences of the hsp60 fragment were aligned in MAFFT [23] (http://www.ebi.ac.uk/Tools/msa/mafft/). The phylogenetic tree was generated by MEGA X [24].
WGS
To determine the draft whole-genome sequences of 95 ECC clinical isolates (Table 1), genomic DNA was extracted from bacteria using DNeasy Blood & Tissue Kit (Qiagen, Hulsterweg, The Netherlands), and DNA library for WGS was prepared using Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA) according to the manufacturer’s instructions. The libraries were sequenced on MiSeq Sequencing System (Illumina) with 300 bp paired-end reads according to the manufacturer’s protocol.
Table 1.
Species determined by ANI, hsp60 cluster classification, and CST susceptibility of ECC clinical isolates
|
hsp60 cluster |
ECC species (No. of isolates) | |
|---|---|---|
| CST heteroresistant (38) | CST susceptible (57) | |
| I | E. asburiae (7), E. cancerogenus (1) | E. asburiae (16), E. soli (1) |
| II | E. kobei (14) | E. kobei (1) |
| III | E. chuandaensisa (1) | E. hormaechei subsp. hoffmannii (2) |
| IV | E. roggenkampii (9) | – |
| V | – | E. ludwigii (15) |
| VI | – | E. hormaechei subsp. xiangfangensis (6), E. hormaechei subsp. oharae (1) |
| VIII | – | E. hormaechei subsp. steigerwaltii (13) |
| IX | E. chuandaensisa (3) | E. chuandaensisa (1) |
| XI | E. cloacae subsp. cloacae (1) | – |
| XII | E. cloacae subsp. dissolvens (2) | – |
| XIII | – | E. mori (1) |
aANI value 94.1%
MLST
MLST was performed using ECC MLST databases in PubMLST.org (https://pubmlst.org/organisms/enterobacter-cloacae/) and the Center for Genomic Epidemiology MLST 2.0 Web tool (https://cge.cbs.dtu.dk/services/MLST/).
ECC species identification
ECC species were identified by ANI analysis using fastANI of the draft WGS data compared with the type strain genomes obtained from GenBank [25]. A cutoff of 98% of the ANI value compared with the type strains was used for species and subspecies identification [7, 26].
Phylogenetic analysis of core genome single nucleotide polymorphisms (SNPs), annotation, and mcr gene identification
Core genome SNPs-based phylogenetic analysis was performed with WGS data. MiSeq read data were mapped to the reference genome, E. cloacae ATCC 13047 (accession number: NC_014121.1), using bwa version 0.7.17. The core genome of the applied strain was extracted using mpileup of SAMtools version 1.9 and the “cns” option of VarScan version 2.3.9 [27, 28]. A phylogenetic tree based on SNPs in the core genome was generated using the default parameter of FastTree version 2.1.10 [29]. Genome annotation was performed with DFAST v1.1.0 and standard settings [30] (https://doi.org/10.1093/bioinformatics/btx713). DNA sequences were compared using Clustal W [31] (https://www.genome.jp/tools-bin/clustalw). mcr genes were identified using assembled genome data by Resfinder (https://www.genomicepidemiology.org).
Reverse transcription quantitative PCR (RT-qPCR)
Overnight cultures with TSB were diluted 1:100 in MHII broth, and cultivated at 37 ℃ for 0, 5, 15, 30, 60, and 120 min in the presence of CST (0, 0.25, 1, 4, 16, and 64 mg/L). Total RNA was extracted using a RNeasy Plus Mini Kit (Qiagen). cDNA was synthesized from total RNA (100 ng) using ReverTra Ace RT-qPCR Master Mix with Genomic DNA (gDNA) Remover (Toyobo). RT-qPCR was performed on LightCycler 480 II system (Roche, Mannheim, Germany) using KOD SYBR qPCR Mix (Toyobo) and primer pairs for arnT, which are shown in Additional file 1: Table S1. Gene expression levels relative to the housekeeping gene gyrB were determined using the ΔΔCt method. Data are expressed as the means ± standard deviations from three independent experiments.
Construction of phoPQ-, eptA-, and arnT-deleted mutants
Deletion mutants of phoPQ, eptA, and arnT were generated by the λ red recombinase method using pKD46-hyg [32, 33]. Mini-cassette genes containing 50 bases corresponding to the upstream and downstream regions of the target genes and kanamycin resistance cassettes (Gene Bridges, Heidelberg, Germany) were amplified by PCR and the gene deletion was confirmed by PCR using specific primers (Additional file 1: Table S1).
Statistical analysis
Mann–Whitney and a one-way ANOVA with the Kruskal–Wallis tests were used to examine the significance of differences between two and multiple groups, respectively. Occurrence rates of phenotypes among groups were examined by χ2-test. p < 0.05 was considered significant. Pearson’s correlation coefficients were also calculated.
Results
Classification of ECC and K. aerogenes clinical isolates into hsp60 clusters
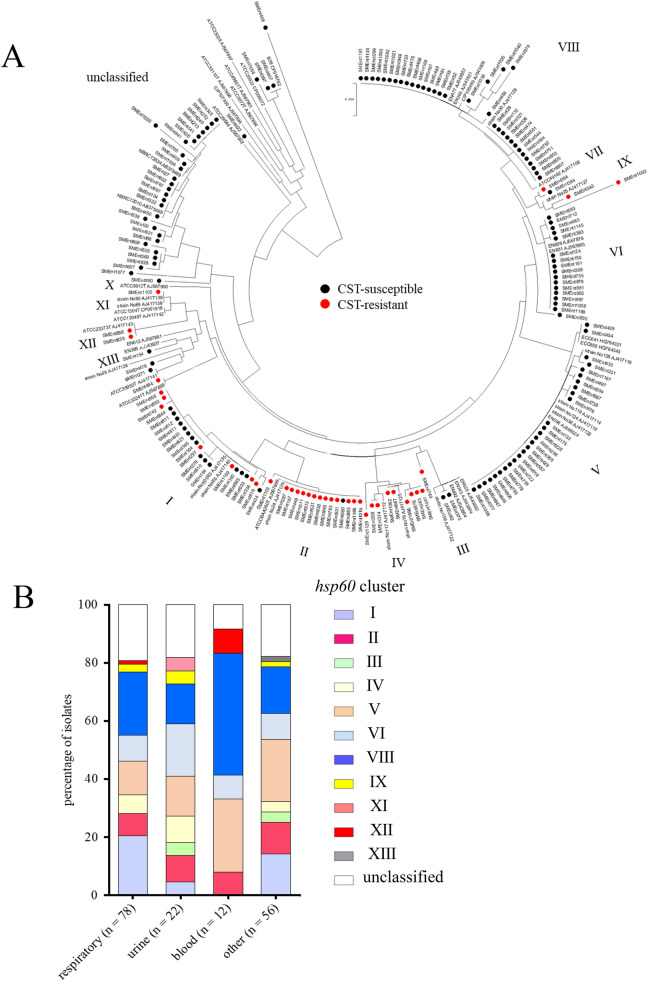
We analyzed ECC isolates and K. aerogenes isolates. Because K. aerogenes was formerly classified in genus Enterobacter, and CST-resistant K. aerogenes was reported [3, 11]. The 138 ECC isolates were classified into 11 hsp60 clusters, and 30 K. aerogenes isolates were defined as unclassified (Fig. 1A, Additional file 1: Table S2). The most abundant cluster was VIII, followed by V, I, and VI (Table 2). There was no significant bias in the distribution of hsp60 cluster types according to the origin of the clinical specimens (Fig. 1B).
Fig. 1.
Classification of ECC and K. aerogenes into hsp60 clusters. A Neighbor-joining unrooted tree based on hsp60 clustering of ECC and K. aerogenes clinical isolates. CST-resistant and -susceptible isolates are indicated by red and black circles, respectively. The GenBank accession numbers of the reference genes are AJ417128 (I), AJ417141 (I), AJ567895 (I), AJ417135 (I), AJ417140 (I), AJ567899 (II), AJ417126 (II), AJ543882 (III), AJ543804 (III), AJ543864 (III), AJ417112 (IV), AJ417125 (IV), AJ866524 (V), AJ417132 (V), AJ417115 (V), AJ417114 (V), AJ417118 (V), HG764321 (V), HG764343 (V), AJ567878 (VI), AJ567885 (VI), AJ417108 (VII), AJ543857 (VIII), AJ543908 (VIII), AJ543821 (VIII), AJ417127 (IX), AJ567900 (X), AJ417138 (XI), AJ417139 (XI), CP001918 (XI), AJ417142 (XI), AJ417143 (XII), AJ567881 (XIII), AJ543837 (XIII), CP016762 (unclassified), CP009072 (unclassified), AJ567897 (unclassified), AJ567901 (unclassified), AJ567902 (unclassified), AJ567894 (unclassified), AJ567898 (unclassified), AJ567896 (unclassified), AB375469 (unclassified), and AB375468 (unclassified). B Comparison of hsp60 cluster types of ECC and K. aerogenes clinical isolates according to the origin of clinical specimens. Significant differences of the occurrence rates of hsp60 clusters did not observe among the origins of specimens
Table 2.
hsp60 cluster classification and prevalence of CST-resistant ECC clinical isolates
| hsp60 cluster | No. of ECC isolates (percentage among ECC isolates) | No. of CST-resistant isolates (percentage among each cluster) |
|---|---|---|
| I | 25 (18.1%) | 8 (32.0%) |
| II | 15 (10.9%) | 14 (93.3%) |
| III | 3 (2.2%) | 1 (33.3%) |
| IV | 9 (6.5%) | 9 (100%) |
| V | 27 (19.6%) | 0 (0%) |
| VI | 17 (12.3%) | 0 (0%) |
| VIII | 34 (24.6%) | 0 (0%) |
| IX | 4 (2.9%) | 3 (75.0%) |
| XI | 1 (0.7%) | 1 (100%) |
| XII | 2 (1.4%) | 2 (100%) |
| XIII | 1 (0.7%) | 0 (0%) |
CST susceptibility of ECC and K. aerogenes, and its relationships with the origin of the clinical specimens and hsp60 clustering
The MIC of CST was determined for the 138 ECC and 30 K. aerogenes clinical isolates (Table 3). It ranged from 0.5 to > 128 mg/L, and MIC50 and MIC90 were 1 and > 128 mg/L, respectively. Thirty-eight (27.5%) of ECC isolates were CST-resistant (MIC ≥ 8 mg/L), whereas none of CST-resistant isolates were observed in K. aerogenes.
Table 3.
CST susceptibility testing of 138 ECC and 30 K. aerogenes clinical isolates
| Bacterial species | CST MIC (mg/ L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | > 128 | |
| No. of ECC isolates | – | – | – | 44 | 56 | – | – | 1 | 2 (2) | 2 (1) | – | 5 | 28 (7) |
| No. of K. aerogenes isolates | – | – | – | 18 | 12 | – | – | – | – | – | – | – | – |
Breakpoint, 2 mg/L of CST. Parentheses indicates No. of isolates displaying the skip-well phenomenon
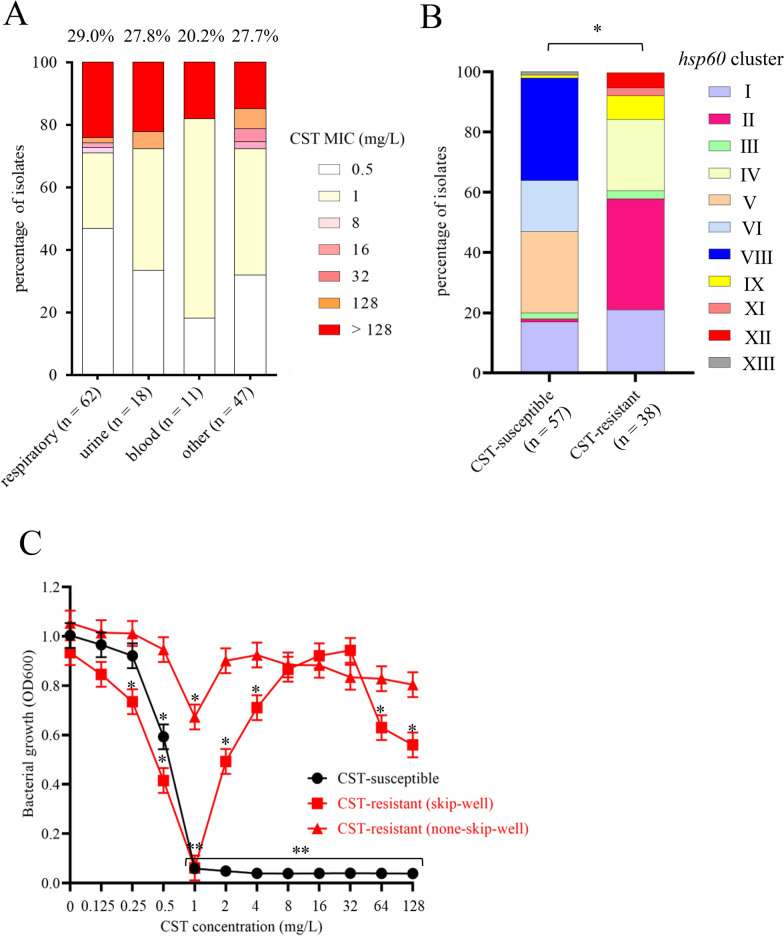
The prevalence of CST-resistant ECC isolates did not significantly differ according to the origin of the clinical specimens (Fig. 2A). The distribution of hsp60 cluster types significantly differed between CST-susceptible and -resistant isolates (p < 0.01) (Fig. 2B). CST-resistant isolates comprised seven clusters, and clusters II, IV, and I were dominant (Table 2). All isolates in clusters IV, XI, and XII, almost all isolates in clusters II and IX, and approximately one-third of isolates in clusters I and III were CST-resistant.
Fig. 2.
Comparison of CST-resistant and -susceptible ECC. A Comparison of CST MICs of ECC clinical isolates according to the origin of clinical specimens. Percentages indicate the prevalence of CST-resistant isolates. Numbers in parentheses indicate the number of ECC isolates. CST susceptibility did not significantly differ according to the origin of clinical isolates. B hsp60 cluster classification of CST-susceptible and -resistant ECC clinical isolates. Significant difference was observed between the groups (*p < 0.05). C Growth of CST-susceptible and -resistant (with and without the skip-well phenomenon) ECC clinical isolates in the presence of various CST concentrations. Bacterial growth was measured by assessing turbidity (OD600) after cultivation for 20 h based on the broth microdilution method in 96-well plates. The skip-well phenomenon was defined as no growth (OD600 < 0.1) at a certain CST concentration(s) and growth (OD600 > 0.1) at higher CST concentrations. Representative ECC clinical isolate data are shown: CST-susceptible isolate (SMEnt52 strain; E. hormaechei subsp. steigerwaltii, hsp60 cluster VIII), CST-resistant isolate displaying the skip-well phenomenon (SMEnt513 strain; E. kobei, hsp60 cluster II), and CST-resistant isolate not displaying the skip-well phenomenon (none-skip-well) (SMEnt1102 strain; E. cloacae subsp. cloacae, hsp60 cluster XI). Bacterial growths (OD600 values) in the presence of CST were compared with the bacterial growth in the absence of CST of the respective strains (*p < 0.05 and **p < 0.001)
Skip-well phenomenon and PAP
Ten CST-resistant isolates exhibited the skip-well phenomenon (26.3% of CST-resistant isolates and 7.2% of total ECC isolates) (Table 3, Additional file 1: Table S2). Their growth was almost completely inhibited in wells containing 0.5 or 1 mg/L CST, but cells grew in wells containing higher CST concentrations (higher than 2 mg/L) (Fig. 2C). Although the remaining 28 CST-resistant isolates did not display the skip-well phenomenon, they exhibited weak growth (OD600 of 0.2–0.6) in wells containing 0.5 or 1 mg/L CST and strong growth (OD600 > 0.8) in wells containing higher CST concentrations (≥ 2 mg/L) (Fig. 2C).
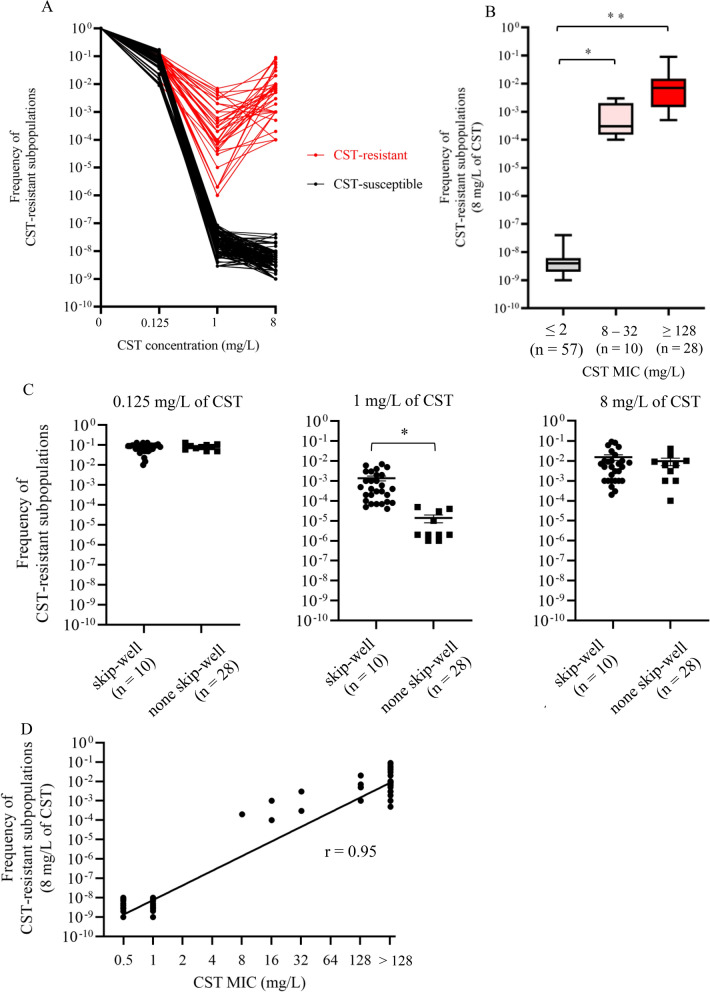
To analyze the CST-heteroresistant phenotype by PAP, we measured the occurrence frequencies of CST-resistant subpopulations in the presence of various CST concentrations (Fig. 3A). Growth of all isolates was unaffected in the presence of 0.125 mg/L CST. For all CST-susceptible isolates, the occurrence frequency of CST-resistant subpopulations was lower than 1 × 10–7 (ranged from 1.6 × 10–9 to 8.5 × 10–8) in the presence of 1 and 8 mg/L CST. For all CST-resistant isolates, the occurrence frequency of CST-resistant subpopulations in the presence of 1 and 8 mg/L CST (more than eightfold higher than 0.125 mg/L) exceeded 1 × 10–7 (ranged from 2.2 × 10–6 to 7.1 × 10–3 at 1 mg/L and from 1.3 × 10–4 to 6.4 × 10–2 at 8 mg/L). These results indicate that all CST-resistant ECC clinical isolates were CST heteroresistant.
Fig. 3.
PAP of ECC. A Occurrence frequencies of CST-resistant subpopulations in the presence of various CST concentrations (0, 0.125, 1, and 8 mg/L). B Occurrence frequencies of CST-resistant subpopulations in isolates with low, moderate, and high CST MICs. Number in parentheses indicates the number of ECC isolates. *p < 0.05 and **p < 0.001. C Comparison of the occurrence frequencies of CST-resistant subpopulations between CST-resistant isolates displaying and not displaying the skip-well phenomenon in the presence of 0.125, 1, and 8 mg/L CST. Number in parentheses indicates the number of ECC isolates. *p < 0.05. D Correlation between CST MICs and occurrence frequencies of CST-resistant subpopulations
The occurrence frequencies of CST-resistant subpopulations in CST-resistant isolates with high (≥ 128 mg/L) and moderate (8–32 mg/L) MICs were significantly higher than those in CST-susceptible isolates (Fig. 3B). They also tended to be higher in CST-resistant isolates with high MICs than in those with moderate MICs, but this difference was not significant. The occurrence frequencies of CST-resistant subpopulations in the presence of 1 mg/L CST were significantly higher in CST-resistant isolates that did not exhibit the skip-well phenomenon than in those that did (p < 0.05) (Fig. 3C), whereas there is no significant differences in 0.125 and 8 mg/L of CST. The occurrence frequencies of CST-resistant subpopulations correlated with the CST MICs (r = 0.95) (Fig. 3D).
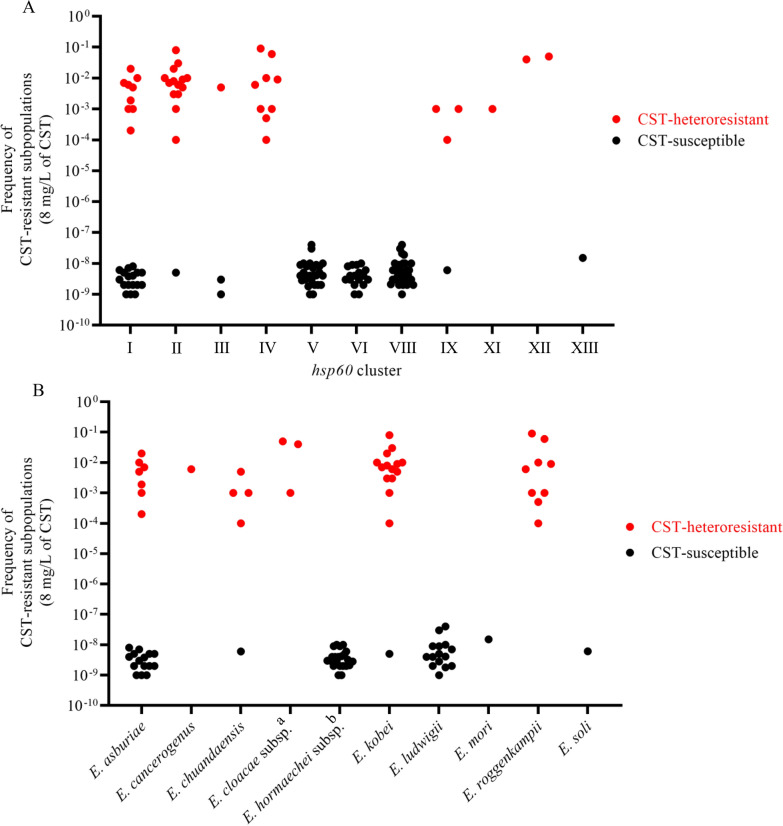
We evaluated the relationship between the occurrence frequencies of CST-resistant subpopulations and hsp60 cluster types. In agreement with the results of CST susceptibility testing (Table 3), CST-heteroresistant isolates with higher occurrence frequencies of CST-resistant subpopulations (> 1 × 10–5) were found in certain hsp60 clusters (I, II, III, IV, IX, XI, and XII) but not in other hsp60 clusters (p < 0.001) (Fig. 4A), and in certain species, such as E. kobei, E. roggenkampii, and E. cloacae subsp. (p < 0.001) (Fig. 4B).
Fig. 4.
ECC species and occurrence frequencies of CST-resistant subpopulations. A Occurrence frequencies of CST-resistant subpopulations according to hsp60 cluster classification. B Occurrence frequencies of CST-resistant subpopulations according to ECC species determined by ANI. CST-heteroresistant and -susceptible isolates are indicated by red and black circles, respectively. aE. cloacae subsp. including E. cloacae subsp. cloacae (n = 1) and E. cloacae subsp. dissolvens (n = 2). bE. hormaechei subsp. including E. hormaechei subsp. hoffmannii (n = 2), E. hormaechei subsp. oharae (n = 1), E. hormaechei subsp. steigerwaltii (n = 13), and E. hormaechei subsp. xiangfangensis (n = 6)
MLST analysis and antimicrobial susceptibilities of CST-heteroresistant ECC clinical isolates
MLST analysis of the CST-heteroresistant isolates was performed. Sequence type (ST)s, ST1939, ST125, ST997, and ST1953 were the dominant in hsp60 clusters I, II, IV, and XII, respectively. In antimicrobial susceptibility testing, 12 (31.6%) CST-heteroresistant isolates were resistant to cefotaxime and one was resistant to meropenem (Additional file 1: Table S3). No CST-heteroresistant isolate was resistant to aminoglycosides and ciprofloxacin.
ECC species identification by ANI and generation of a phylogenetic tree based on core genome SNPs
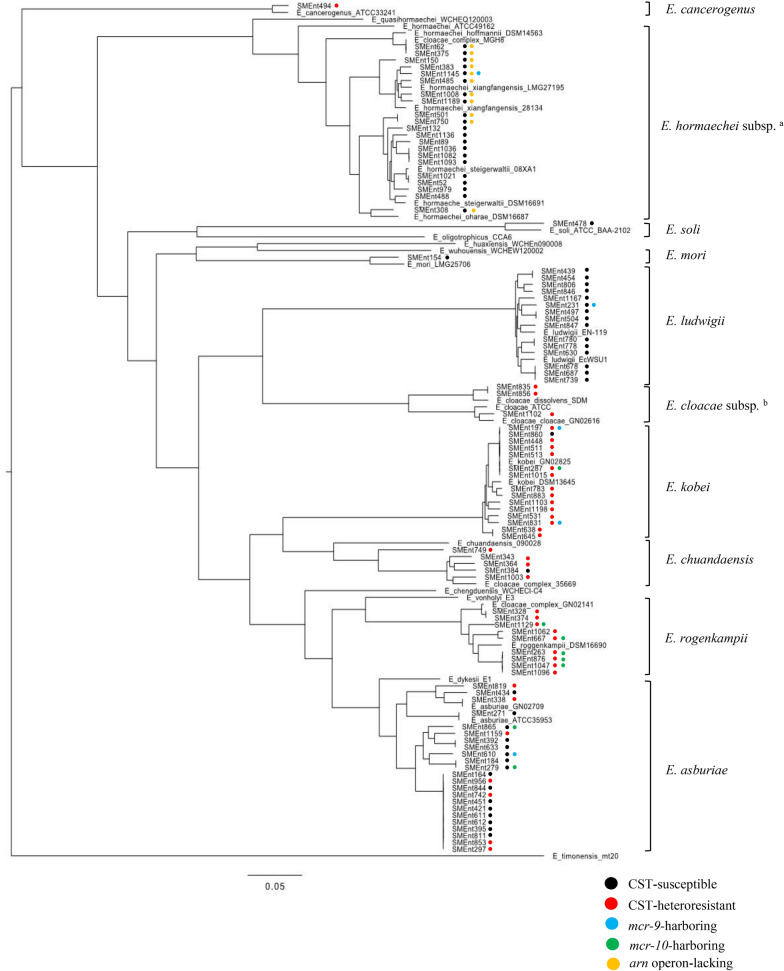
Ninety-five clinical isolates identified as ECC by the MALDI Biotyper were analyzed by ANI, and a phylogenetic tree was generated using core genome SNPs (Fig. 5, Additional file 1: Table S2). All 38 CST-heteroresistant isolates and 57 CST-susceptible isolates randomly selected to include half the isolates in each hsp60 cluster were examined. Ten species were identified by ANI (Additional file 1: Table S2). The CST-heteroresistant isolates were identified as one of six species: E. asburiae, E. cancerogenus, E. chuandaensis, E. cloacae (including subsp. cloacae and subsp. dissolvens), E. kobei, and E. roggenkampii (Table 1). The 57 CST-susceptible isolates were identified as one of seven species: E. asburiae, E. chuandaensis, E. hormaechei (including subsp. hoffmannii, subsp. oharae, subsp. steigerwaltii, and subsp. xiangfangensis), E. kobei, E. ludwigii, E. mori, and E. soli (Table 1). The occurrence frequencies of CST-resistant subpopulations were closely related with the results of species identification, phylogeny, and hsp60 clustering (Fig. 4A, B).
Fig. 5.
Phylogenetic tree based on core genome SNPs of ECC. Core genome SNP-based phylogenetic analysis was performed with WGS data of 95 ECC clinical isolates. CST-susceptible strains, CST-heteroresistant strains, strains harboring mcr-9, strains harboring mcr-10, and strains lacking the arn operon are indicated by black, red, blue, green, and yellow circles to the right of their names. aE. hormaechei (subsp. hoffmannii, subsp. xiangfangensis, subsp. steigerwaltii, and subsp. oharae). bE. cloacae (subsp. cloacae and subsp. dissolvens). The GenBank accession numbers of the reference genes are NZ_CP011863 (E. asburiae), NZ_LEDH00000000 (E. asburiae), NZ_FYBA00000000 (E. cancerogenus), NZ_CP043318 (E. chengduensis), NZ_QZCS00000000 (E. chuandaensis), NC_014121 (E. cloacae subsp. cloacae), NZ_LEDL00000000 (E. cloacae subsp. cloacae), NC_018079 (E. cloacae subsp. dissolvens), NZ_VTTY00000000 (E. dykesii), NZ_CP017186 (E. hormaechei subsp. hoffmannii), NZ_AFHR01000000 (E. hormaechei subsp. hormaechei), NZ_CP017180 (E. hormaechei subsp. oharae), NZ_CP017179 (E. hormaechei subsp. steigerwaltii), NZ_AMGJ01000000 (E. hormaechei subsp. steigerwaltii), NZ_CP017183 (E. hormaechei subsp. xiangfangensis), NZ_LABA01000000 (E. hormaechei subsp. xiangfangensis), NZ_CP043342 (E. huaxiensis), NZ_CP01718 (E. kobei), NZ_LEDC01000000 (E. kobei), NZ_JTLO00000000 (E. ludwigii), NC_016514 (E. ludwigii), NZ_GL890773 (E. mori), NZ_AP019007 (E. oligotrophicus), NZ_SJON00000000 (E. quasihormaechei), NZ_CP017184 (E. roggenkampii), NZ_LXES00000000 (E. soli), NZ_FCOP00000000 (E. timonensis), NZ_VTUC00000000 (E. vonholyi), NZ_SJOO00000000 (E. wuhouensis), NZ_JZZB01000000 (ECC), NZ_LEEP01000000 (ECC), and NZ_AXLJ01000000 (ECC)
In CST-susceptible ECC, 10 isolates of E. hormaechei subsp. (including subsp. hoffmannii, subsp. oharae, subsp. steigerwaltii, and subsp. xiangfangensis) lacked the arn operon in their genomes according to annotation analysis, whereas other chromosomal CST resistance determinants (phoPQ, mgrB, pmrAB, and eptA) were present (Fig. 5). mcr-9 and mcr-10 were detected in five isolates (two E. kobei and one each of E. asburiae, E. hormaechei subsp. xiangfangensis, and E. ludwigii) and eight isolates (five E. roggenkampii, two E. asburiae, and one E. kobei), respectively (Fig. 5). The CST MIC and occurrence frequency of CST-resistant subpopulations analyzed by PAP did not significantly differ among the none-mcr, mcr-9-positive, and mcr-10-positive groups (Additional file 1: Table S4, Additional file 2: Fig. S1).
arnT mRNA expression levels in CST-heteroresistant and -susceptible ECC clinical isolates
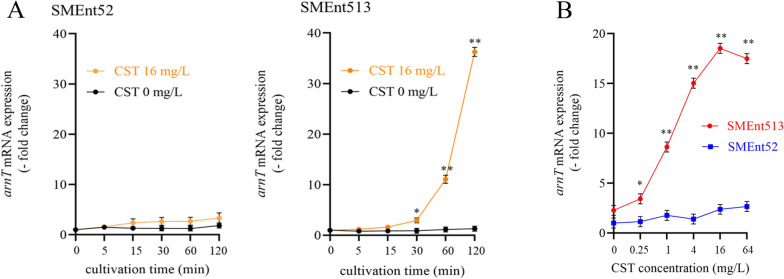
We measured the arnT mRNA expression levels in the CST-heteroresistant strain SMEnt513 (E. kobei, hsp60 cluster II which mostly includes CST-heteroresistant isolates) and the CST-susceptible strain SMEnt52 (E. hormaechei subsp. steigerwaltii, hsp60 cluster VIII which mostly includes CST susceptible isolates) in the presence and absence of CST. CST induced arnT mRNA expression in SMEnt513, but not in SMEnt52 (Fig. 6A). arnT mRNA expression in SMEnt513 was significantly increased by treatment with 0.25 mg/L CST and peaked (approximately 18-fold) upon treatment with 16 mg/L CST for 45 min (Fig. 6B).
Fig. 6.
arnT mRNA expression levels in CST-susceptible and -heteroresistant ECC. SMEnt52 (E. hormaechei subsp. steigerwaltii, hsp60 cluster VIII) and SMEnt513 (E. kobei, hsp60 cluster II) were used as CST-susceptible and -resistant isolates, respectively. A arnT mRNA expression levels during cultivation of SMEnt52 and SMEnt513 in the presence (16 mg/L) and absence of CST. The y axis shows relative expression levels normalized by that at 0 min (set to 1.0). *p < 0.05 and **p < 0.001. B arnT mRNA expression levels in SMEnt52 and SMEnt513 cultivated in the presence of various CST concentrations. Total RNA was isolated after cultivation for 45 min at 37 ℃. The y axis shows relative mRNA expression levels normalized by that in SMEnt52 in the absence of CST (set to 1.0). *p < 0.05 and **p < 0.001
Evaluation of the CST heteroresistance mechanism in ECC
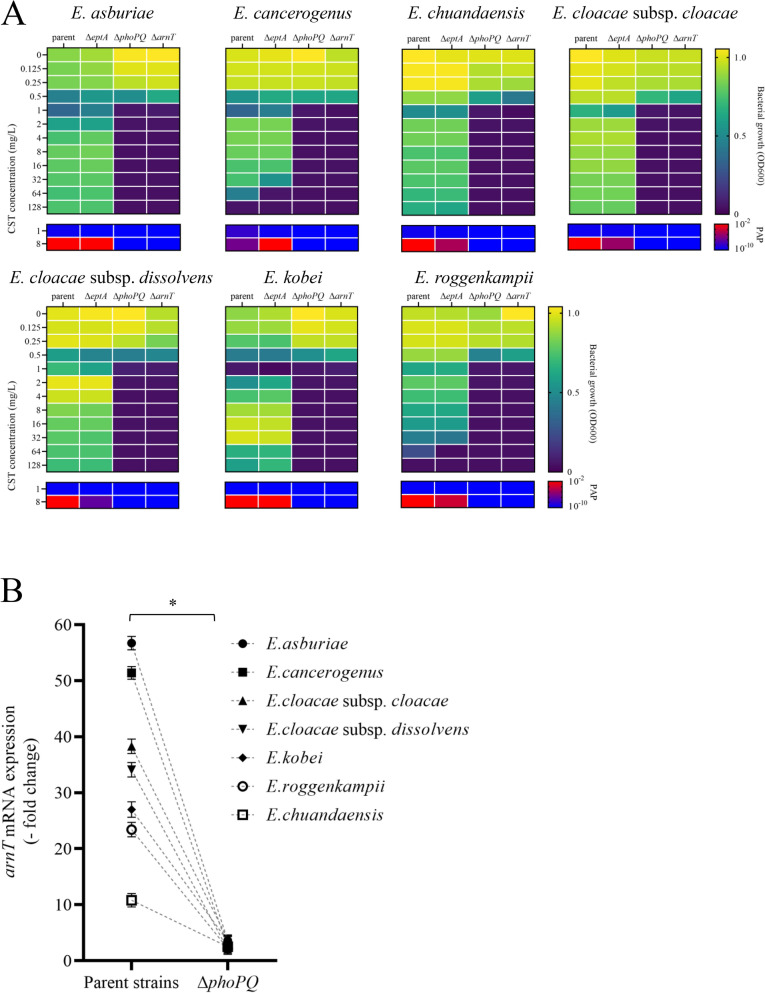
To evaluate the mechanism underlying CST heteroresistance in individual ECC species, we selected one CST-heteroresistant strain from each of the seven species. No mutation involved in CST resistance was found in phoPQ, mgrB, pmrAB, arnT, and eptA between these CST-heteroresistant and CST-susceptible isolates. We generated phoPQ-, eptA-, and arnT-deleted mutants of the seven isolates. All parent strains and eptA-deleted mutants exhibited the typical CST-heteroresistant phenotype in the presence of CST (growth was decreased in the presence of 0.5 or 1 mg/L CST but was observed in the presence of higher CST concentrations) (Fig. 7A). All phoPQ- and arnT-deleted mutants did not grow in the presence of ≥ 1 mg/L CST. In PAP analysis, the occurrence frequencies of CST-resistant subpopulations were high (> 1 × 10–3) in parent strains and eptA-deleted mutants in the presence of 8 mg/L CST, but were markedly decreased (< 1 × 10–8) and below the criteria of heteroresistance (ranged from 1 × 10–7 to 1 × 10–1) in phoPQ- and arnT-deleted mutants (Fig. 7A). In the susceptibility test, the phenotype of phoPQ- and arnT-deleted mutants changed from CST-resistant to CST-susceptible (Additional file 1: Table S5). Treatment with 16 mg/L CST induced arnT mRNA expression more than tenfold in the parent strains of all ECC species, whereas arnT mRNA induction by CST was abolished in all phoPQ-deleted mutants (Fig. 7B).
Fig. 7.
CST-heteroresistant phenotype of phoPQ-, eptA-, and arnT-deleted mutants derived from various species of ECC. Deletion mutants were prepared for the following seven representative species of ECC clinical isolates: SMEnt819 (E. asburiae by ANI, hsp60 cluster I), SMEnt494 (E. cancerogenus, hsp60 cluster I), SMEnt1102 (E. cloacae subsp. cloacae, hsp60 cluster XI), SMEnt835 (E. cloacae subsp. dissolvens, hsp60 cluster XII), SMEnt513 (E. kobei, hsp60 cluster II), SMEnt1062 (E. roggenkampii, hsp60 cluster IV), and SMEnt1003 [E. chuandaensis (ANI value 94.1%), hsp60 cluster IX]. A Heat maps representing bacterial growth and occurrence of CST-resistant subpopulations in the presence of CST. Bacterial growth was measured by assessing turbidity (OD600) after cultivation for 20 h based on the broth microdilution method in 96-well plates. OD600 < 0.1 was defined as no growth. A CST MIC ≥ 4 mg/L was defined as CST resistance. The occurrence frequencies of CST-resistant subpopulations were measured in the presence of 1 or 8 mg/L CST. Color shading was used to visualize the turbidities (OD600) and frequencies of CST-resistant subpopulations at each CST concentration. Turbidities (OD600) decrease from yellow to purple and occurrence frequencies of CST-resistant subpopulations in PAP decrease from red to blue. B Comparison of arnT mRNA expression levels in the presence of CST (16 mg/L) between parent strains and their phoPQ-deleted mutants. The y axis shows relative mRNA expression levels normalized by those in the absence of CST in each strain (set to 1.0). *p = 0.002
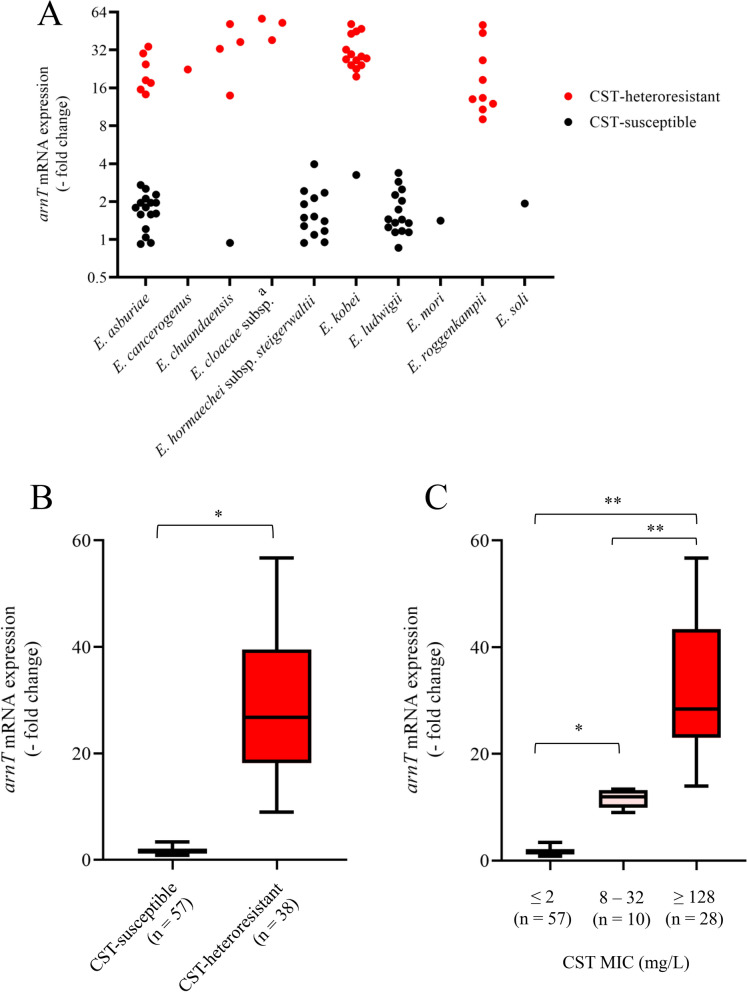
In the presence of 16 mg/L CST, arnT mRNA expression levels were increased more than eightfold (9.1–56.7-fold) in all CST-heteroresistant isolates, but less than fourfold in CST-susceptible isolates (Fig. 8A, B). The arnT mRNA expression levels were positively related with CST MICs (Fig. 8C).
Fig. 8.
Induction of arnT mRNA expression in the presence of CST in ECC. A arnT mRNA induction by CST in various species found in ECC clinical isolates. The y axis shows relative mRNA expression levels following culture in the presence of 16 mg/L CST for 120 min. The values were normalized by those in the absence of CST in each strain (set to 1.0). CST-heteroresistant and -susceptible isolates are indicated by red and black circles, respectively. Isolates of E. hormaechei subsp. hoffmannii, E. hormaechei subsp. oharae, and E. hormaechei subsp. xiangfangensis, which lack the arn operon, were not examined. a, E. cloacae subsp. cloacae (n = 1) and E. cloacae subsp. dissolvens (n = 2). B Comparison of arnT mRNA induction by CST between CST-susceptible and -heteroresistant ECC clinical isolates. The y axis shows relative mRNA expression levels in ECC clinical isolates cultured in the presence of 16 mg/L CST for 120 min. Number in parentheses indicates the number of ECC isolates. The values were normalized by those in the absence of CST in each strain (set to 1.0). *p < 0.001. C arnT mRNA induction by CST in ECC clinical isolates with low, moderate, and high CST MICs. The y axis shows relative mRNA expression levels in the presence of 16 mg/L CST normalized by those in the absence of CST in each strain (set to 1.0). Number in parentheses indicates the number of ECC isolates. *p < 0.05 and **p < 0.001
Discussion
In the present study, 27.5% of ECC clinical isolates obtained from a university hospital in Japan was CST resistant showing heteroresistant phenotype. In the previous studies, the prevalence of CST resistance in ECC clinical isolates was 23.9% in Korea (2012–2014) [11], 11.3–13.3% in UK and Ireland (2017) [10], and 34.5% in China (2020) [34]. These observations indicate that CST-resistant ECC is present in clinical specimens of various origins. Although no multidrug-resistant ECC was detected in our study, the CST resistance in ECC should be an international concern because multidrug-resistant ECC has emerged [9]. However, CST-heteroresistance phenotype has not been adequately examined for the CST-resistant isolates in previous studies [9–11, 16, 17, 34].
The CST-heteroresistant isolates were assigned to certain species and lineages similar to a previous French report [6]. However, no CST-heteroresistant isolates were found in clusters V and VIII in the present study, whereas 100% and 79% of the clusters were CST-heteroresistant in the report, respectively [6] suggesting different CST-heteroresistant lineages have been spread in other counties.
We found several mcr-9/10-positive isolates in various lineages regardless of CST susceptibility. Acquisition of the plasmid-mediated CST resistance genes mcr-9 and mcr-10 contributed little to CST heteroresistance in ECC because CST MICs and occurrence frequencies of CST-resistant subpopulations did not obviously differ between mcr-9/10-positive and -negative isolates. This observation strongly indicates that CST heteroresistance of ECC was mainly mediated by intrinsic genes, namely, CST-inducible arnT, not by exogenous genes.
The STs of CST-heteroresistant isolates were diverse, but E. asburiae ST1939 (hsp60 cluster I), E. kobei ST125 (hsp60 cluster II), and E. roggenkampii ST997 (hsp60 cluster IV) accounted for more than 40% of each species. E. kobei ST125 and E. roggenkampii ST997 have been clinically isolated in many countries [35–38]. These findings suggest that certain CST-heteroresistant ECC lineages have spread worldwide.
We evaluated the skip-well phenomenon with the broth microdilution method, which is expected to be an easy and useful approach to screen heteroresistant isolates [11]. However, only 26.3% of CST-heteroresistant isolates exhibited the skip-well phenomenon. The isolates with a higher occurrence frequency of CST-resistant subpopulations (from 1 × 10–4 to 1 × 10–2) by PAP did not display the skip-well phenomenon because this frequency exceeded the inoculating cell number used in the susceptibility test of 5 × 105 CFU/mL (5 × 104 CFU/100 µL in each well of a 96-well plate). Thus, monitoring the skip-well phenomenon was an unreliable method to detect CST heteroresistance in ECC clinical isolates. PAP is a gold standard for detecting heteroresistance [19], but is laborious. We found CST-heteroresistant isolates grew less or not at all at a certain CST concentration (0.5 or 1 mg/L) and grew proficiently at higher CST concentrations. Thus, measuring bacterial growth by assessing turbidity (OD600) in the presence of various CST concentrations should be convenient method for screening CST-heteroresistant isolates in routine clinical laboratory. It did not require any additional experiments other than those performed to determine CST MICs by the broth microdilution method (Additional file 1: Table S2).
Why do CST-resistant subpopulations appear? The present study demonstrated that the PhoPQ-arnT pathway was involved in CST heteroresistance in certain ECC species, E. asburiae, E. cancerogenus, E. chuandaensis, E. cloacae (subsp. cloacae and subsp. dissolvens), E. kobei, and E. roggenkampii. Contribution of this pathway has been shown in a reference strain E. cloacae subsp. cloacae ATCC 13047 [16]. In addition, we revealed that this pathway is activated by CST, and the resulting arnT induction occurred in the presence of CST at a concentration around the MIC. Thus, CST-heteroresistant phenomenon, namely decreased bacterial growth in the presence of 0.5 or 1 mg/L CST, was attributed to inadequate (or unresponsive) arnT induction at lower CST concentrations (< 2 mg/L), while induced ArnT overcame the bactericidal activity of CST at higher CST concentrations (≥ 2 mg/L). These findings suggest that the appearance of CST-resistant subpopulations in a strain displaying CST heteroresistance reflects the ability of arnT induction by CST.
Conclusion
This study demonstrates that CST-heteroresistant ECC is present in clinical specimens from various infection sites. Inducible CST heteroresistance is mediated via the PhoPQ-arnT pathway in CST-resistant isolates of certain ECC species, such as E. roggenkampii, E. kobei, E. chuandaensis, E. cloacae, and a part of E. asburiae. On the other hand, no CST-resistant isolates were found in E. hormaechei and E. ludwigii. Therefore, attention is required to be paid to CST heteroresistance at all infection sites of these ECC species, which have a common intrinsic gene background, such as species, hsp60 clustering, MLST, and/or core genomic features. Exogenous mcr genes, such as mcr-9 and mcr-10, found in ECC clinical isolates [34, 39, 40] contribute less to CST resistance.
Supplementary Information
Additional file 1: Table S1. Sequences of primers used in this study. Table S2. Characteristics of ECC and K. aerogenes clinical isolates used in this study. Table S3. Characteristics of CST-heteroresisatnt ECC clinical isolates. Table S4. CST MICs of none-mcr, mcr-9-positive, and mcr-10-positive ECC isolates. Table S5. Comparison of CST susceptibility of phoPQ-, eptA-, and arnT-deleted mutants of different ECC species.
Additional file 2: Fig. S1. Contribution of mcr-9 and mcr-10 to CST heteroresistance of ECC clinical isolates. A Comparison of the occurrence frequencies of CST-resistant subpopulations among none-mcr, mcr-9-positive, and mcr-10-positive ECC clinical isolates. There was no significant difference among the groups. B–G Comparison of the occurrence frequencies of CST-resistant subpopulations between mcr-9-positive and -negative B–D and between mcr-10-positive and -negative E–G isolates. These isolates were selected from the same node of the core genome phylogeny. PAP was performed with the following CST concentrations: 0.125 (B and E), 1 (C and F), and 8 (D and G) mg/L. There were no significant differences among the groups. a, E. hormaechei subsp. xiangfangensis.
Acknowledgements
Not applicable.
Abbreviations
- ANI
Average nucleotide identity
- CLSI
Clinical and Laboratory Standards Institute
- CST
Colistin
- ECC
Enterobacter cloacae complex
- hsp60
Heat shock protein 60 gene
- L-Ara4N
4-Amino-4-deoxy-l-arabinose
- MALDI-TOF MS
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- MHII agar
Mueller–Hinton II agar
- MHII broth
Mueller–Hinton II broth
- MIC
Minimum inhibitory concentration
- MLST
Multilocus sequence typing
- PAP
Population analysis profiling
- pEtN
Phosphoethanolamine
- RT-qPCR
Reverse transcription quantitative PCR
- SNPs
Single nucleotide polymorphisms
- ST
Sequence type
- TSB
Trypticase soy broth
- WGS
Whole-genome sequencing
Author contributions
SF participated in experimental works, statistical analyses, and preparing the manuscript. TS conceived the objectives and designed the study protocol and revised the manuscript. KA performed ANI, MLST, and core genome SNPs-based phylogenetic analysis. SYamamoto and NO made kind discussion, and revised the manuscript. TS, CN and YS conducted WGS MH supervised the study ST collected the clinical isolates. SY supervised the study, critically revised and edited the manuscript. All authors approved the final manuscript.
Funding
This work was supported by the Japan Agency for Medical Research and Development (AMED) (JP20ak0101118h0002, JP223fa627005, JP22wm0125008, and 22jk021004h0001), JSPS KAKENHI (JP21H03622), Takeda Science Foundation, the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), and the Joint Research Program of the International Institute for Zoonosis Control, Hokkaido University. All funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank databases under accession numbers from DRX398430 to DRX398524.
Declarations
Ethics approval and consent to participate
This study was approved by the Sapporo Medical University Hospital Institutional Review Board (IRB No. 272-70).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Sanders WE, Jr, Sanders CC. Enterobacter spp.:pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997;10:220–241. doi: 10.1128/CMR.10.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davin-Regli A, Lavigne JP, Pagès JM. Enterobacter spp.: update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin Microbiol Rev. 2019;32:e00002–19. doi: 10.1128/CMR.00002-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu W, Wei L, Feng Y, Kang M, Zong Z. Enterobacter huaxiensis sp. nov. and Enterobacter chuandaensis sp. nov, recovered from human blood. Int J Syst Evol Microbiol. 2019;69:708–714. doi: 10.1099/ijsem.0.003207. [DOI] [PubMed] [Google Scholar]
- 5.Sutton GG, Brinkac LM, Clarke TH, Fouts DE. Enterobacter hormaechei subsp. hoffmannii subsp. nov., Enterobacter hormaechei subsp. xiangfangensis comb. nov., Enterobacter roggenkampii sp. nov., and Enterobacter muelleri is a later heterotypic synonym of Enterobacter asburiae based on computational analysis of sequenced Enterobacter genomes. F1000Research. 2018;7:521. doi: 10.12688/f1000research.14566.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guérin F, Isnard C, Sinel C, Morand P, Dhalluin A, Cattoir V, et al. Cluster-dependent colistin hetero-resistance in Enterobacter cloacae complex. J Antimicrob Chemother. 2016;71:3058–3061. doi: 10.1093/jac/dkw260. [DOI] [PubMed] [Google Scholar]
- 7.Kim M, Oh HS, Park SC, Chun J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol. 2014;64:346–351. doi: 10.1099/ijs.0.059774-0. [DOI] [PubMed] [Google Scholar]
- 8.Davin-Regli A, Pagès JM. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front Microbiol. 2015;6:392. doi: 10.3389/fmicb.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annavajhala MK, Gomez-Simmonds A, Uhlemann AC. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol. 2019;10:44. doi: 10.3389/fmicb.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mushtaq S, Reynolds R, Gilmore MC, Esho O, Adkin R, García-Romero I, et al. Inherent colistin resistance in genogroups of the Enterobacter cloacae complex: epidemiological, genetic and biochemical analysis from the BSAC resistance surveillance programme. J Antimicrob Chemother. 2020;75:2452–2461. doi: 10.1093/jac/dkaa201. [DOI] [PubMed] [Google Scholar]
- 11.Hong YK, Lee JY, Ko KS. Colistin resistance in Enterobacter spp. isolates in Korea. J Microbiol. 2018;56:435–440. doi: 10.1007/s12275-018-7449-0. [DOI] [PubMed] [Google Scholar]
- 12.Aghapour Z, Gholizadeh P, Ganbarov K, Bialvaei AZ, Mahmood SS, Tanomand A, et al. Molecular mechanisms related to colistin resistance in Enterobacteriaceae. Infect Drug Resist. 2019;12:965–975. doi: 10.2147/IDR.S199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Srinivas S, Xu Y, Wei W, Feng Y. Genetic and biochemical mechanisms for acterial lipid A modifiers associated with polymyxin resistance. Trends Biochem Sci. 2019;44:973–988. doi: 10.1016/j.tibs.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mmatli M, Mbelle NM, Sekyere JO. Global epidemiology, genetic environment, risk factors and therapeutic prospects of mcr genes: a current and emerging update. Front Cell Infect Microbiol. 2022;12:941358. doi: 10.3389/fcimb.2022.941358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang KN, Klein DR, Kazi MI, Guérin F, Cattoir V, Brodbelt JS, et al. Colistin heteroresistance in Enterobacter cloacae is regulated by PhoPQ-dependent 4-amino-4-deoxy-l-arabinose addition to lipid A. Mol Microbiol. 2019;111:1604–1616. doi: 10.1111/mmi.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Band VI, Crispell EK, Napier BA, Herrera CM, Greg K, Vavikolanu K, et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat Microbiol. 2017;1:16053. doi: 10.1038/nmicrobiol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nicoloff H, Hjort K, Levin BR, Andersson DI. The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat Microbiol. 2019;4:504–514. doi: 10.1038/s41564-018-0342-0. [DOI] [PubMed] [Google Scholar]
- 19.Andersson DI, Nicoloff H, Hjort K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat Rev Microbiol. 2019;17:479–496. doi: 10.1038/s41579-019-0218-1. [DOI] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute . M100-Performance standards for antimicrobial susceptibility testing. 31. Wayne: Clinical and Laboratory Standards Institute; 2021. [Google Scholar]
- 21.Sato T, Harada K, Usui M, Yokota S, Horiuchi M. Colistin susceptibility in companion animal-derived Escherichia coli, Klebsiella spp., and Enterobacter spp. in Japan: frequent isolation of colistin-resistant Enterobacter cloacae complex. Front Cell Infect Microbiol. 2022;12:946841. doi: 10.3389/fcimb.2022.946841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann H, Roggenkamp A. Population genetics of the nomenspecies Enterobacter cloacae. Appl Environ Microbiol. 2003;69:5306–5318. doi: 10.1128/AEM.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katoh K, Rozewicki J, Yamada KD. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 2018;20:1160–1166. doi: 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun. 2018;9:5114. doi: 10.1038/s41467-018-07641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chavda KD, Chen L, Fouts DE, Sutton G, Brinkac L, Jenkins SG, et al. Comprehensive genome analysis of carbapenemase-producing Enterobacter spp.: new insights into phylogeny, population structure, and resistance mechanisms. MBio. 2016;7:e02093–e2116. doi: 10.1128/mBio.02093-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Price MN, Dehal PS, Arkin AP. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanizawa Y, Fujisawa T, Kaminuma E, Nakamura Y, Arita M. DFAST and DAGA: web-based integrated genome annotation tools and resources. Biosci Microbiota, Food Health. 2016;35:173–184. doi: 10.12938/bmfh.16-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Suzuki Y, Shiraishi T, Honda H, Shinagawa M, Yamamoto S, et al. Tigecycline nonsusceptibility occurs exclusively in fluoroquinolone-resistant Escherichia coli clinical isolates, including the major multidrug-resistant lineages O25b:H4-ST131-H30R and O1-ST648. Antimicrob Agents Chemother. 2017;61:e01654–e1716. doi: 10.1128/AAC.01654-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao W, Cui Y, Quan J, Zhao D, Han X, Shi Q, et al. High prevalence of colistin resistance and mcr-9/10 genes in Enterobacter spp. in a tertiary hospital over a decade. Int J Antimicrob Agents. 2022;59:106573. doi: 10.1016/j.ijantimicag.2022.106573. [DOI] [PubMed] [Google Scholar]
- 35.Jousset AB, Oueslati S, Bernabeu S, Takissian J, Creton E, Vogel A, et al. False-positive carbapenem-hydrolyzing confirmatory tests due to ACT-28, a chromosomally encoded AmpC with weak carbapenemase activity from Enterobacter kobei. Antimicrob Agents Chemother. 2019;63:e02388–e2418. doi: 10.1128/AAC.02388-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu T, Xue CX, Chen Y, Huang J, Wu W, Lu Y, et al. Frequent convergence of mcr-9 and carbapenemase genes in Enterobacter cloacae complex driven by epidemic plasmids and host incompatibility. Emerg Microbes Infect. 2022;11:1959–1972. doi: 10.1080/22221751.2022.2103456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wand ME, Mark SJ. Mutations in the two component regulator systems PmrAB and PhoPQ give rise to increased colistin resistance in Citrobacter and Enterobacter spp. J Med Microbiol. 2020;69:521–529. doi: 10.1099/jmm.0.001173. [DOI] [PubMed] [Google Scholar]
- 38.Mori N, Kagawa N, Aoki K, Ishi Y, Tateda K, Aoki Y. Clinical and molecular analyses of bloodstream infections caused by IMP metallo-β-lactamase-producing Enterobacteriaceae in a tertiary hospital in Japan. J Infect Chemother. 2020;26:144–147. doi: 10.1016/j.jiac.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmanna M. Identification of novel mobilized colistin resistance gene mcr- 9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. MBio. 2019;10:e00853–e919. doi: 10.1128/mBio.00853-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect. 2020;9:508–516. doi: 10.1080/22221751.2020.1732231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Sequences of primers used in this study. Table S2. Characteristics of ECC and K. aerogenes clinical isolates used in this study. Table S3. Characteristics of CST-heteroresisatnt ECC clinical isolates. Table S4. CST MICs of none-mcr, mcr-9-positive, and mcr-10-positive ECC isolates. Table S5. Comparison of CST susceptibility of phoPQ-, eptA-, and arnT-deleted mutants of different ECC species.
Additional file 2: Fig. S1. Contribution of mcr-9 and mcr-10 to CST heteroresistance of ECC clinical isolates. A Comparison of the occurrence frequencies of CST-resistant subpopulations among none-mcr, mcr-9-positive, and mcr-10-positive ECC clinical isolates. There was no significant difference among the groups. B–G Comparison of the occurrence frequencies of CST-resistant subpopulations between mcr-9-positive and -negative B–D and between mcr-10-positive and -negative E–G isolates. These isolates were selected from the same node of the core genome phylogeny. PAP was performed with the following CST concentrations: 0.125 (B and E), 1 (C and F), and 8 (D and G) mg/L. There were no significant differences among the groups. a, E. hormaechei subsp. xiangfangensis.
Data Availability Statement
Nucleotide sequence data reported are available in the DDBJ/EMBL/GenBank databases under accession numbers from DRX398430 to DRX398524.