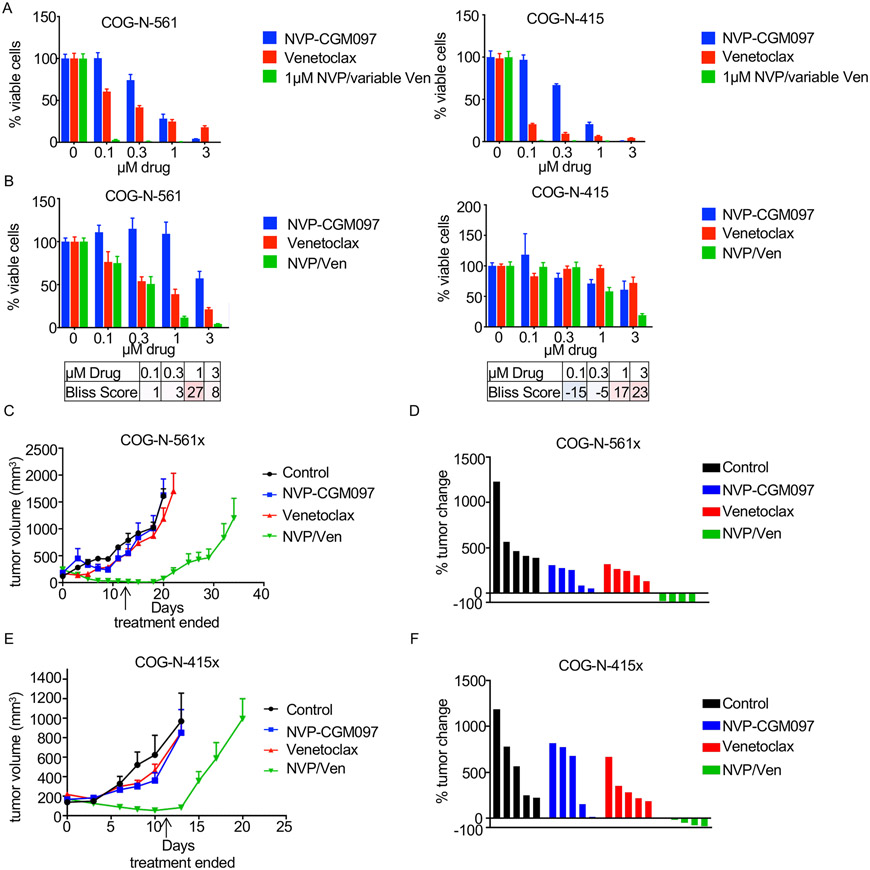
Figure 3. NVP-CGM097 and venetoclax effective in vivo.
(A) Cell viability assays were performed with MYCN-amplified NB ex vivo cell lines corresponding to PDX models treated for 72h with increasing NVP-CGM097, increasing venetoclax, or increasing venetoclax in combination with 1μM NVP-CGM097. (B) Cell viability assays were performed with MYCN-amplified NB ex vivo cell lines corresponding to PDX models treated for 16h with NVP-CGM097, venetoclax, or the combination at the indicated concentrations with bliss synergy scores shown. Red indicates synergy, blue indicates antagonism. (C) The COG-N-561x PDX model was treated with NVP-CGM097, venetoclax, or the combination of both drugs 5 days/week for two weeks and tumor size was monitored for 35 days. (D) Percent change of tumor volume on the day treatment ended for the COG-N-561x PDX model. (E) The COG-N-415x PDX model was treated with NVP-CGM097, venetoclax, or the combination of both drugs 5 days/week for two weeks and tumor size was monitored for 20 days. (F) Percent change of tumor volume on the day treatment ended for the COG-N-415x PDX model.