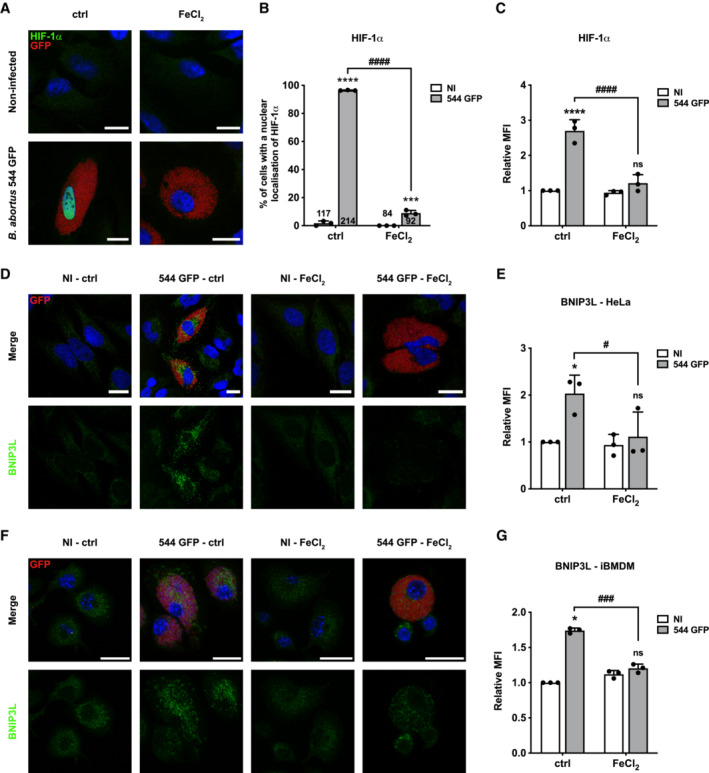
Figure 4. Iron prevents B. abortus‐induced HIF‐1α/BNIP3L pathway activation in HeLa cells and iBMDM.
-
ARepresentative confocal micrographs of HeLa cells infected or not with B. abortus 544 GFP (red) treated or not (ctrl) with FeCl2 (500 μM) for 48 h, then fixed and immunostained for HIF‐1α (Alexa 568—green). DNA was stained with Hoechst 33258 (blue). Scale bars: 20 μm.
-
BQuantification of the percentages of cells positive for a nuclear localisation of HIF‐1α from HeLa cells infected or not (NI) with B. abortus 544 GFP (red) and treated or not (ctrl) with 500 μM FeCl2 for 48 h from micrographs shown in (A). Data are presented as means ± SD from n = 3 (biological replicates) independent experiments (the numbers indicated in the columns represent the number of cells analysed per condition). Statistical analyses were performed using a two‐way ANOVA followed by a Šidàk's multiple comparisons test; asterisks indicate significant differences compared to the control (NI); ***P < 0.001; ****P < 0.0001; hashtags indicate significant differences compared to the infected condition without FeCl2; #### P < 0.0001.
-
CRelative median fluorescence intensity (MFI) of HIF‐1α immunostaining from HeLa cells infected or not (NI) with B. abortus 544 GFP treated or not (ctrl) with 500 μM FeCl2 for 48 h as measured by flow cytometry. Data are presented as means ± SD from n = 3 independent experiments (10,093 cells analysed in total per condition). Statistical analyses were performed using a two‐way ANOVA followed by a Šidàk's multiple comparisons test; asterisks indicate significant differences compared to the control (NI); ns, not significant; ****P < 0.0001; hashtags indicate significant differences compared to the infected condition without FeCl2; #### P < 0.0001.
-
DRepresentative confocal micrographs of HeLa cells infected or not (NI) with B. abortus 544 GFP (red) treated or not (ctrl) with 500 μM FeCl2 for 48 h, then fixed and immunostained for BNIP3L (Alexa 568—green). DNA was stained with Hoechst 33258 (blue). Scale bars: 20 μm.
-
ERelative median fluorescence intensity (MFI) of BNIP3L immunostaining from HeLa cells infected or not (NI) with B. abortus 544 GFP treated or not (ctrl) with 500 μM FeCl2 for 48 h as measured by flow cytometry. Data are presented as means ± SD from n = 3 (biological replicates) independent experiments (10,199 cells analysed in total per condition). Statistical analyses were performed using a two‐way ANOVA followed by a Šidàk's multiple comparisons test; asterisks indicate significant differences compared to the control (NI); ns, not significant; *P < 0.05; hashtags indicate significant differences compared to the infected condition without FeCl2; # P < 0.05.
-
FRepresentative confocal micrographs of iBMDM infected or not (NI) with B. abortus 544 GFP (red) treated or not (ctrl) with 500 μM FeCl2 for 48 h, then fixed and immunostained for BNIP3L (Alexa 568—green). DNA was stained with Hoechst 33258 (blue). Scale bars: 20 μm.
-
GRelative median fluorescence intensity (MFI) of BNIP3L immunostaining from iBMDM infected or not (NI) with B. abortus 544 GFP treated or not (ctrl) with 500 μM FeCl2 for 48 h as measured by flow cytometry. Data are presented as means ± SD from n = 3 (biological replicates) independent experiments (14,152 cells analysed in total per condition). Statistical analyses were performed using a two‐way ANOVA followed by a Šidàk's multiple comparisons test; asterisks indicate significant differences compared to the control (NI); ns, not significant; *P < 0.05; hashtags indicate significant differences compared to the infected condition without FeCl2; ### P < 0.001.
Source data are available online for this figure.
