Fig. 1.
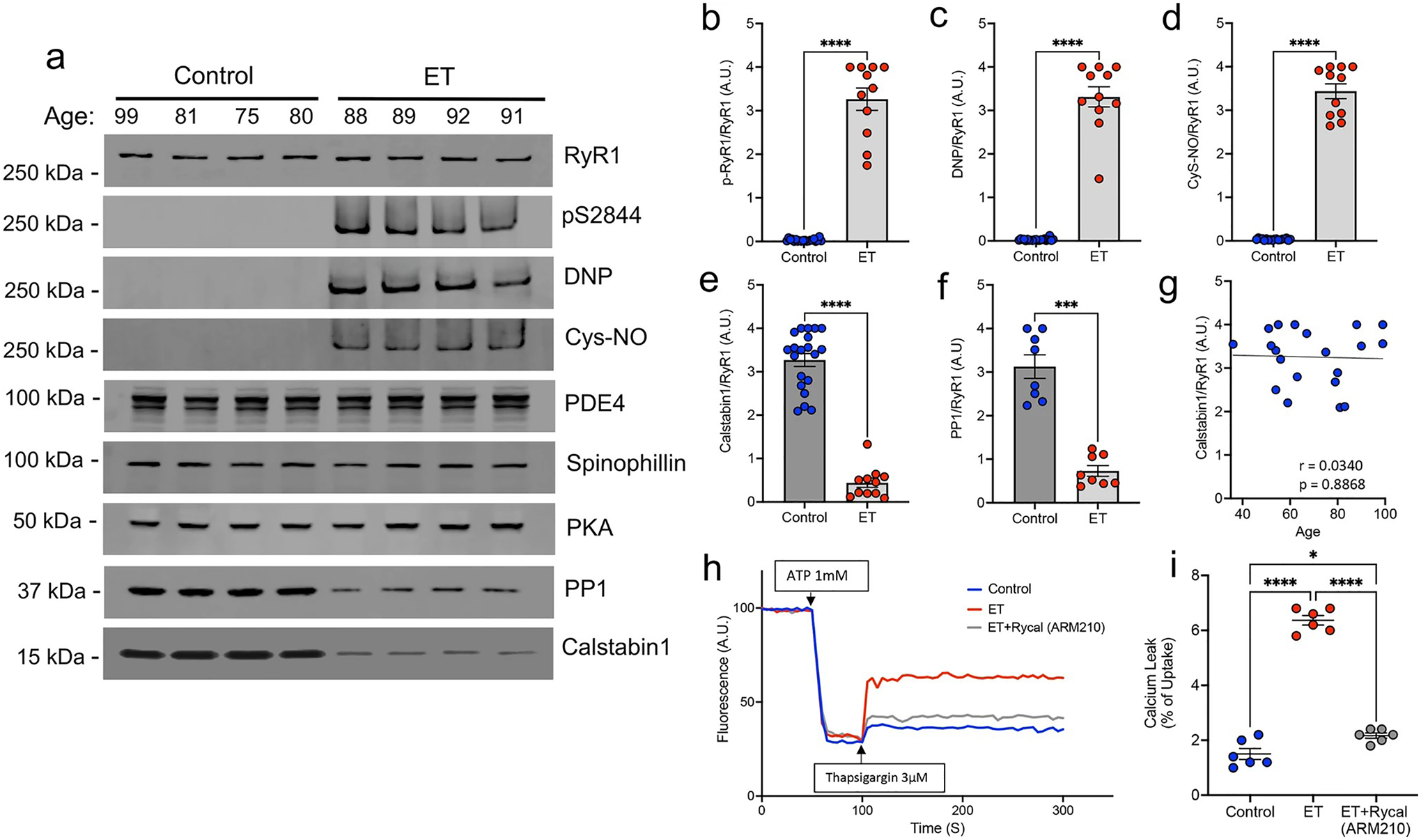
RyR1 channels are ‘leaky’ in ET cerebellar cortex. a Representative western blot of immunoprecipitated RyR1 macromolecular complex probing for total RyR1, RyR1-pS2844, oxidation (DNP), nitrosylation (Cys-NO), and other RyR1 interactors, PDE4, Spinophillin, PKA, PP1 and Calstabin1. Quantification of protein levels normalized to total RyR1 in n = 20 controls and n = 11 ETs for b p-RyR1/RyR1, c DNP/RyR1, d Cys-NO/RyR1, e Calstabin1/RyR1, and f PP1/RyR1 [Mann–Whitney t test ****p < 0.0001]. g Calstabin1/RyR1 vs. age in control cerebellar samples only, aged 36–99 (n = 20), demonstrating that normal aging does not significantly increase calstabin1 depletion from RyR1. h Representative graph of ER calcium (Ca2+) leak measured in microsomes isolated from postmortem control and ET cerebellum, demonstrating increased Ca2+ leak in ET versus control. Pharmacologically inhibiting ER Ca2+ leak by in vitro incubation of ET microsomes with RyCal (ARM210), an RyR1 channel stabilizer, prior to the assay attenuates the Ca2+ leak. i. Quantification of microsomal calcium release assays. Bar graph represents the leak (after thapsigargin) as a percentage of the calcium update after ATP (n = 6). [One Way ANOVA w/Tukey’s multiple comparisons ****p < 0.0001, *p < 0.05]
