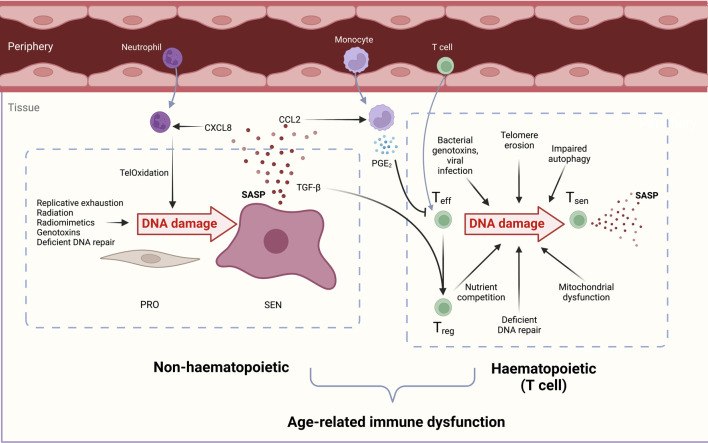
FIGURE 2.
Overview of the causes and consequences of DNA damage in both haematopoietic (T cell) and non-haematopoietic cells which contribute to immunosenescence. DNA-damaged immune and non-haematopoietic cell senescence are linked. During ageing, senescent cells increase in both compartments due to reduced senescent cell clearance, deficient DNA repair processes, and increased genotoxin abundance. Non-haematopoietic cells: Proliferating cells (PRO) experience genotoxicity from radiation, excessive replication, chemotherapeutic drugs, and radiomimetics to drive persistent DNA damage, proliferation arrest, and cellular senescence (SEN). The senescence-associated secretory phenotype (SASP) contains chemoattractants such as CXCL8 that draw in peripheral neutrophils. These induce oxidative damage in stromal cell telomeric DNA, called TelOxidation, propagating further paracrine senescence. The SASP component CCL2 attracts monocytes which secrete PGE2 and suppress T cell functions. TGF-β in the SASP favours Treg generation and a pro-suppressive, inadequate immune response during ageing. Haematopoietic cells: Effector T cells (Teff) experience genotoxic injury from bacterial genotoxins viral infections short telomeres, reactive oxygen species (ROS) from dysfunctional mitochondria, and nutrient competition with regulatory T cells (Tregs). DNA-damaged T cells manifest senescent phentotypes in a sestrin- and ATM-dependent manner. Senescent T cells (Tsen) are dysfunctional and contribute to inflammageing with their inflammatory SASP, tissue cytotoxicity, and immune dysfunction during ageing.