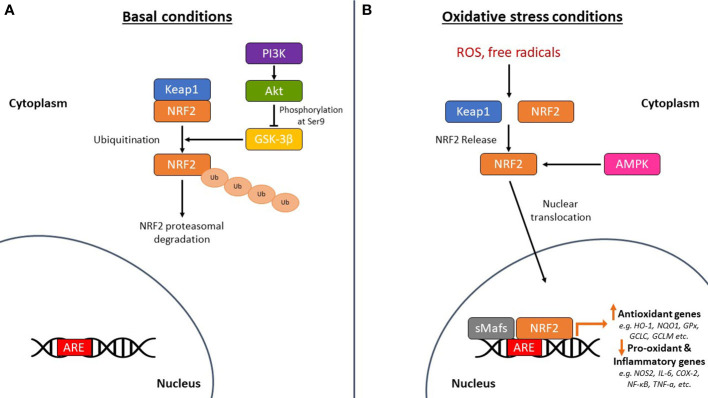
Figure 2.
The mechanism of action of nuclear factor erythroid-derived 2-related factor 2 (NRF2) in basal and oxidative stress conditions. (A) Under basal conditions, NRF2 is sequestered by Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm leading to its ubiquitination and subsequent proteasomal degradation. Glycogen synthase kinase-3β (GSK-3β) phosphorylates NRF2 leading again to the proteasomal degradation of NRF2. The PI3K (phosphoinositide-3 kinase)/Akt (protein kinase B) signaling pathway phosphorylates GSK-3β at serine 9 to keep it in an inactive state, thereby protecting NRF2 from proteasomal degradation. (B) Under oxidative stress conditions, NRF2 is released from Keap1 and is translocated to the nucleus where it dimerizes with small musculoaponeurotic fibrosarcoma proteins (sMafs) and binds to specific DNA regions, the antioxidant response element (ARE) sequences. This regulates the expression of antioxidant genes, such as heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione peroxidase (GPx), glutamate-cysteine ligase catalytic subunit (GCLC), and glutamate-cysteine ligase regulatory subunit (GCLM). It also downregulates the expression of pro-oxidant and pro-inflammatory genes, such as nitric oxide synthase 2 (NOS2), interleukin (IL)-6, cyclooxygenase 2 (COX-2), nuclear factor kappa beta (NF-κB), and tumor necrosis factor-a (TNF-a). 5’-Adenosine monophosphate-activated protein kinase (AMPK) is also linked to the phosphorylation and direct activation of NRF2 nuclear translocation, tuning the transactivation of antioxidant genes. ROS, reactive oxygen species; Ub, ubiquitin.