Abstract
Background and Objectives
β-Amyloid (Aβ) plaques can co-occur with Lewy-related pathology in patients with dementia with Lewy bodies (DLB), but Aβ load at prodromal stages of DLB still needs to be elucidated. We investigated Aβ load on PET throughout the DLB continuum, from an early prodromal stage of isolated REM sleep behavior disorder (iRBD) to a stage of mild cognitive impairment with Lewy bodies (MCI-LB), and finally DLB.
Methods
We performed a cross-sectional study in patients with a diagnosis of iRBD, MCI-LB, or DLB from the Mayo Clinic Alzheimer Disease Research Center. Aβ levels were measured by Pittsburgh compound B (PiB) PET, and global cortical standardized uptake value ratio (SUVR) was calculated. Global cortical PiB SUVR values from each clinical group were compared with each other and with those of cognitively unimpaired (CU) individuals (n = 100) balanced on age and sex using analysis of covariance. We used multiple linear regression testing for interaction to study the influences of sex and APOE ε4 status on PiB SUVR along the DLB continuum.
Results
Of the 162 patients, 16 had iRBD, 64 had MCI-LB, and 82 had DLB. Compared with CU individuals, global cortical PiB SUVR was higher in those with DLB (p < 0.001) and MCI-LB (p = 0.012). The DLB group included the highest proportion of Aβ-positive patients (60%), followed by MCI-LB (41%), iRBD (25%), and finally CU (19%). Global cortical PiB SUVR was higher in APOE ε4 carriers compared with that in APOE ε4 noncarriers in MCI-LB (p < 0.001) and DLB groups (p = 0.049). Women had higher PiB SUVR with older age compared with men across the DLB continuum (β estimate = 0.014, p = 0.02).
Discussion
In this cross-sectional study, levels of Aβ load was higher further along the DLB continuum. Whereas Aβ levels were comparable with those in CU individuals in iRBD, a significant elevation in Aβ levels was observed in the predementia stage of MCI-LB and in DLB. Specifically, APOE ε4 carriers had higher Aβ levels than APOE ε4 noncarriers, and women tended to have higher Aβ levels than men as they got older. These findings have important implications in targeting patients within the DLB continuum for clinical trials of disease-modifying therapies.
Patients with dementia with Lewy bodies (DLB) commonly have β-amyloid (Aβ) at autopsy, one of the pathologic hallmarks of Alzheimer disease (AD).1-3 Amyloid pathology in patients with DLB can progressively build up during life starting from a prodromal stage to dementia.4,5 One of the prodromal DLB syndromes defined by the current research criteria is mild cognitive impairment with core clinical features of DLB (MCI-LB).6 Increasing evidence has demonstrated that isolated REM sleep behavior disorder (iRBD) may constitute the first manifestation of underlying Lewy-related pathology and is often the first clinically identifiable stage in the DLB continuum occurring before MCI-LB.7
Aβ can be studied in vivo with PET imaging using 11C-Pittsburgh compound B (PiB), one of the most widely investigated Aβ PET ligands. More than half of the patients with probable DLB have elevated Aβ on PiB-PET scan.8-10 A higher PiB-PET uptake in patients with DLB has been associated with greater clinical severity and accelerated disease progression.8,11 An earlier study has also reported a higher PiB-PET uptake in patients with MCI-LB5; however, PiB-PET uptake along the DLB continuum, including iRBD, MCI-LB, and DLB still needed to be elucidated.
Another important question is whether Aβ accumulation in the DLB continuum is associated with factors, such as sex, APOE ε4 status, or core clinical features of DLB. Recent diagnostic criteria for DLB and for MCI-LB are characterized as possible or probable based on diagnostic certainty associated with the number of core clinical features of DLB and presence of indicative biomarkers.6,12 These features are associated with the progression to dementia with underlying Lewy-related pathology13-17; however, the literature about the association of clinical features of DLB with AD pathologic changes, such as cortical Aβ accumulation, is scarce. Determining Aβ load on PET at different stages throughout the DLB continuum and understanding the factors that modify the Aβ load is critical to improve patient selection for new clinical trials designed to target AD-related Aβ deposition in DLB and to enable interpretation of therapeutic response to future interventions targeting alpha-synuclein–related pathology.
Our objective was to determine the Aβ deposition on PET within the DLB continuum, starting from the earliest prodromal stages of iRBD, and MCI-LB, and finally DLB. We also investigated the contribution of sex, APOE ε4 status,, and number of DLB clinical features to variations in Aβ burden. We hypothesized that PiB uptake and frequency of Aβ positivity would be higher in patients in the DLB continuum compared with cognitively unimpaired (CU) controls and that it would increase progressively throughout the disease stages from iRBD to MCI-LB and DLB. We also hypothesized that there would be differences in Aβ PET by sex, APOE ε4 status, and number of DLB clinical features along the DLB continuum.
Methods
Participants
This study included patients with iRBD (n = 16), MCI-LB (n = 64), and probable DLB (n = 82) who were enrolled in the Mayo Clinic Alzheimer Disease Research Center. For this study, we selected patients who had a PET scan with 11C-PiB between August 2007 and August 2021. All patients underwent a medical history review, informant interview, neurologic examination, and neuropsychological assessment. Core clinical features of DLB are parkinsonism, fluctuations, visual hallucinations, and RBD.12 We used the Classification of Sleep Disorders-II edition criteria for the diagnosis of isolated RBD, indicating dream enactment behavior during sleep without current neurologic features and having RBD without atonia confirmed by polysomnography.18 Diagnosis of MCI-LB was made according to the McKeith criteria,6 which require impairment in 1 or more cognitive domains, preserved or minimally affected performance of complex activities of daily living, and 1 or more core clinical feature of DLB. Indicative biomarkers were not used in the diagnosis because of incomplete data on biomarkers. Data of dopamine transporter availability in the putamen on 123I-FP-CIT SPECT (DaTscan), one of the key imaging biomarkers of Lewy body disease, were available only for 37 (58%) patients with MCI-LB. Therefore, in this study, MCI-LB diagnosis relied on a designation of possible MCI-LB for those with 1 core DLB feature and probable MCI-LB for those with 2 or more core DLB features. A diagnosis of clinically probable DLB was based on the 2005 International Consortium Criteria.19 We used 2005 rather than 2017 Consortium Criteria because clinical evaluations occurred before the publication of the new Consortium Criteria in 2017. CU participants (n = 100) without cognitive, motor, or sleep disorders were selected as a control group from the Mayo Clinic Study of Aging (MCSA). In MCSA, participants were characterized by a consensus conference of neurologists, neuropsychologists, and nurse/coordinators based on clinical and neurocognitive assessments. Participants were characterized as CU when they were judged to have no cognitive impairment according to published criteria20,21 and received a score of 0 on the Clinical Dementia Rating (CDR) scale. In all cases, diagnosis and clinical assessments were made blinded to PiB-PET biomarker results.
Details regarding the clinical assessment of cognitively normal participants22 and patient groups6,12 have been provided in previous reports. In brief, parkinsonism was based on the neurologic examination as having at least 2 of the 4 cardinal features (tremors, rigidity, bradykinesia, and postural instability). The Unified Parkinson disease Rating Scale Part III was used to quantify the severity of parkinsonism but was not used in the diagnostic process. Visual hallucinations had to be fully formed, not restricted to a single episode, and not related to another medical issue, dementia, or treatment. The 4-item Mayo Fluctuation Scale was used to determine the presence of cognitive fluctuations. Finally, probable RBD was considered to be present when sleep symptoms met the International Classification of Sleep Disorders-II, diagnostic criteria B. We used the Mini-Mental State Examination (MMSE)23 to assess patients' global cognitive status and CDR Sum of Boxes (CDR-SOB)24 to assess dementia severity.
Standard Protocol Approvals, Registrations, and Patient Consents
The Mayo Clinic Institutional Review Board approved the study, and informed consent on participation was obtained from all patients or an appropriate surrogate, as stipulated in the Declaration of Helsinki.
PiB-PET Imaging and MRI
PET imaging was performed on General Electric PET/CT (GE Healthcare, Chicago, IL) and Siemens PET/CT scanners (Siemens Healthcare, Erlangen, Germany). Differences in scanners and manufacturers were accounted for by following Joshi et al. method for reducing between-scanner differences in multicenter PET studies. PiB scans consisted of four 5-minute dynamic frames after a 40-minute 11C-PiB uptake period. The PET scanning procedures have been previously described.25 PiB-PET images were rigidly aligned to each individual's T1-weighted MRI. T1-weighted MRI examinations were performed at 3T MRI for anatomic segmentation and labeling.
PiB-PET Image Processing and Analysis
An automated image processing pipeline was used to analyze PiB-PET images. In brief, PET images were rigidly aligned to individual's T1-weighted MRIs using statistical parametric mapping 12 (SPM12). MRIs were previously segmented with Unified Segmentation in SPM12 using population-optimized priors from the Mayo Clinic Adult Lifespan Template (MCALT).26 The global PiB retention standardized uptake value ratio (SUVR) was obtained for a standardized meta-region including the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus cortical regions. PiB SUVR was referenced to the median value of cerebellar crus. Patients were further classified as amyloid positive if they had a global cortical PiB SUVR value equal or greater than 1.48, which is derived as previously described.27
A voxel-based analysis was also conducted comparing PiB SUVR in each group against CU and between groups. Analysis was conducted in MCALT space using SPM12. Maps of significant comparisons were displayed at p < 0.05 level after correcting for multiple comparisons using false discovery rate (FDR) error correction.
Statistical Analysis
We report demographic and clinical characteristics using mean values and SDs by clinical groups for continuous variables and counts and percentages for categorical variables. Overall, group differences for continuous variables were analyzed with 1-way analysis of covariance (ANCOVA) and logistic regression with an adjustment for age, except when model convergence issues arouse where Firth penalized logistic regression models were performed. All pairwise group comparisons were performed with Tukey honest significant differences, except for the penalized Firth results where contrast statements were performed. PiB SUVR was analyzed with a log transformation because of skewness. In the DLB continuum group of patients (iRBD + MCI-LB + DLB), we summarized demographic and clinical characteristics by sex and by APOE ε4 carrier status using mean values and SDs for continuous variables and counts and percentages for categorical variables. We examined sex and APOE ε4 status using t tests for continuous variables and χ2 tests for categorical variables. The separately stratified groups of sex and APOE ε4 carrier status were then analyzed within each clinical group for PiB SUVR differences with t tests. Next, we performed multiple linear regression model testing for interactions to examine whether the associations of PiB SUVR with sex and APOE ε4 varied with age in the DLB continuum group of patients. We reduced to parsimonious models with backward elimination when interactions were not significant and produced plots of predicted values. Finally, we performed 1-way ANCOVA, adjusting by age, to compare PiB SUVR between patients with different number of DLB clinical features within the MCI-LB and DLB groups. For the MCI-LB group, the number of clinical features was coded as 1, 2, and 3+ when patients showed 1, 2, and 3 or more clinical features, respectively. PiB SUVR may be different in patients with MCI-LB with 1 core clinical feature of DLB and with abnormal levels in biomarkers of Lewy body pathology when compared with CU individuals because these patients should be classified as probable MCI-LB according to the current criteria of MCI-LB.6 However, DaTscan findings as a biomarker for underlying Lewy body pathology was available only for 58% of patients with MCI-LB. Therefore, we performed a complementary sensitivity analysis to test whether results are different when we include only patients with probable MCI-LB. In this analysis, patients with MCI-LB with 1 clinical feature of DLB and abnormal DaTscan patients were reclassified as probable MCI and added to the group of patients with MCI-LB who had 2 or more core clinical features of DLB (n = 45). Information about DaTscan is detailed in eAppendix 1 (links.lww.com/WNL/C805). We compared PiB SUVR between this new group of probable MCI-LB and CU individuals using ANCOVA, adjusting by age. For the DLB group, the number of core clinical features was coded as 2, 3, and 4 because by definition, probable DLB requires 2 or more core features. Statistical significance was p < 0.05 (2-tailed) in all the analyses.28
Data Availability
Data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Characteristics of the Cohort
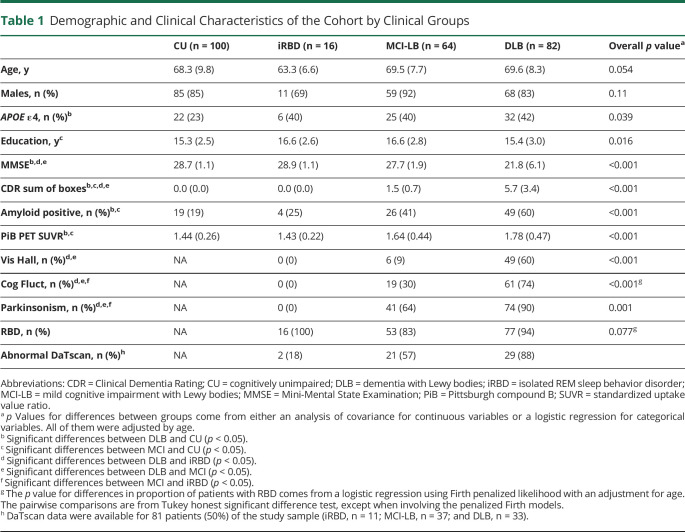
Demographic and clinical characteristics of the cohort by groups are listed in Table 1. All patients with iRBD, and most of the patients with MCI-LB and DLB who had a clinical history of recurrent dream enactment behavior, had confirmation of REM sleep without atonia ± dream enactment behavior during video polysomnography (PSG). Groups did not differ in the proportion of men and women. Patients with iRBD were comparable with CU individuals in all demographic and clinical variables. The iRBD, MCI-LB, and DLB groups were similar in age and sex. Patients with DLB had lower MMSE scores and higher CDR-SOB scores than CU individuals, those with iRBD, and those with MCI-LB. By definition, the DLB group had a higher frequency of visual hallucinations, cognitive fluctuations, and parkinsonism than the iRBD group. The DLB group also had a higher frequency of visual hallucinations, cognitive fluctuations, and parkinsonism than the MCI-LB group. The proportion of APOE ε4 carriers was also higher in the DLB group compared with that in CU individuals. Data on DaTscan were available for 81 (50%) individuals. Among them, more than half of individuals with MCI-LB (21/57; 57%) and DLB (29/33; 88%) had abnormal DaTscan, while a smaller proportion (2/11; 18%) of individuals with iRBD had abnormal DaTscan.
Table 1.
Demographic and Clinical Characteristics of the Cohort by Clinical Groups
Aβ PET Findings
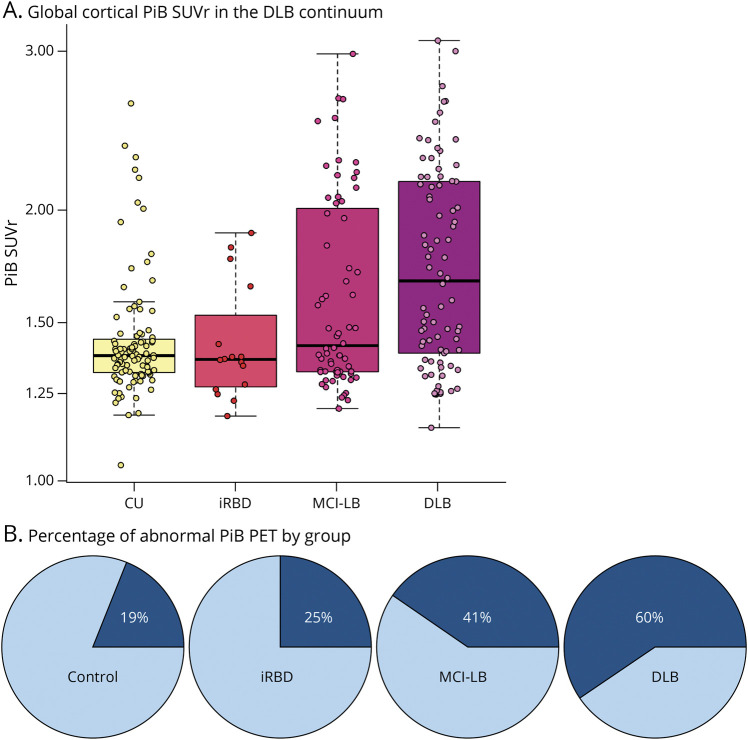
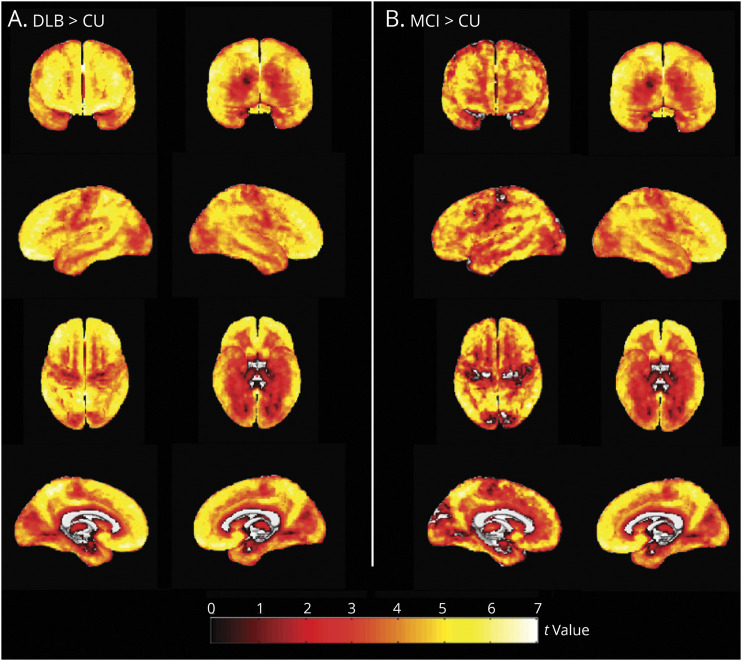
Figure 1 shows the differences in global cortical PiB SUVR and frequency of Aβ positivity among the CU, iRBD, MCI-LB, and DLB groups. Global cortical PiB SUVR was higher in DLB (p < 0.001) and MCI-LB (p = 0.012) groups compared with that in the CU group (Table 1; Figure 1A). The DLB group also had a higher global cortical PiB SUVR compared with the iRBD group, but this did not reach statistical significance (p = 0.051). Global cortical PiB SUVR did not differ between patients with iRBD and CU individuals (p = 0.92), nor between those with iRBD and MCI-LB (p = 0.66), or between those with DLB and MCI-LB (p = 0.10). The proportion of patients classified as Aβ positive also differed between groups (p < 0.001; Table 1). The DLB group included the highest proportion of Aβ-positive patients (60%), followed by those with MCI-LB (41%), those with iRBD (25%), and finally CU individuals (19%) (Figure 1B). The proportion of Aβ positivity was higher in those with DLB compared with that in CU individuals (p < 0.001) and in individuals with MCI-LB compared with that in CU individuals (p = 0.014). Voxel-based differences in PiB SUVR of those with DLB and MCI-LB compared with that in CU individuals are displayed in Figure 2. The DLB and MCI-LB groups had greater PiB uptake showing a similar pattern in the entire cortex compared with the CU group (p < 0.05) after correction for FDR.
Figure 1. Global Cortical Aβ Accumulation in the DLB Continuum.
(A) Global cortical 11C-PiB SUVR. (B) the percentage of Aβ positivity among the clinical groups in the DLB continuum. Aβ = β-amyloid; CU = cognitively unimpaired; DLB = dementia with Lewy bodies; iRBD = isolated REM sleep behavior disorder; MCI-LB = mild cognitive impairment with Lewy bodies; PiB = Pittsburgh compound B; SUVR = standardized uptake value ratio.
Figure 2. Voxel-Based Analysis Comparing 11C-PiB SUVR in the DLB Continuum.
Maps are displayed at the p < 0.05 level with the t values displayed in the color bar. Correction for multiple comparisons was applied with false discovery rate error correction. CU = cognitively unimpaired; DLB = dementia with Lewy bodies; MCI-LB = mild cognitive impairment with Lewy bodies; PiB = Pittsburgh compound B; SUVR = standardized uptake value ratio.
Differences in Aβ PET Findings by Sex and APOE ε4 Status
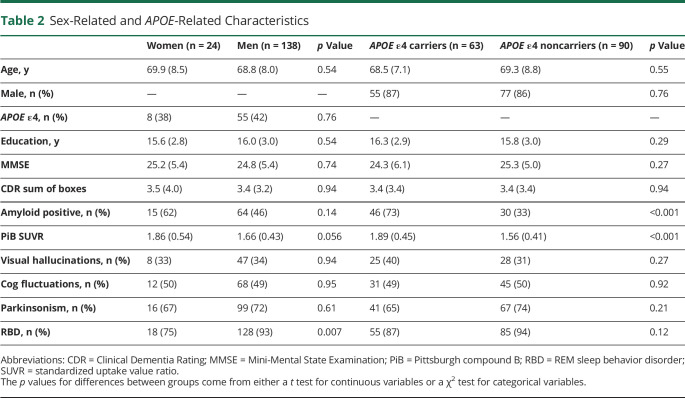
We examined whether global cortical PiB SUVR among groups differed by sex and APOE ε4 carriership. Table 2 summarizes the demographic and clinical characteristics of the DLB continuum group by sex and APOE ε4 status. RBD was more common in men than women (93% vs 75%, p = 0.007; Table 2), but there were no sex differences in other clinical characteristics or APOE ε4 status.
Table 2.
Sex-Related and APOE-Related Characteristics
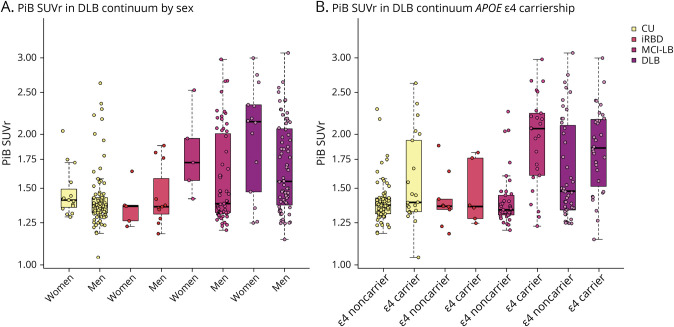
In the DLB continuum, PiB SUVR was associated with APOE ε4 status (p < 0.001) but was not associated with sex (p = 0.056). Multivariable regression analyses yielded an interaction between age and sex (p = 0.02), showing that women tend to have greater increase in PiB SUVR with age (β estimate = 0.014). There was no interaction with APOE ε4 (p = 0.22) status and age. When age and APOE ε4 status were included in the same model, older age (p < 0.001) and APOE ε4 status (p < 0.001) predicted higher PiB SUVR (Figure 3). In a supplemental analysis, we stratified the sample by group, though notably, the number of women in each subgroup was small (n = 5, 31.3% iRBD women; n = 5, 7.8% MCI-LB women; and n = 14, 17.1% DLB women, 15% CU women). Only the DLB group showed a sex difference with women showing higher PiB SUVR than men (p = 0.031), but when age was included in the model, this difference was not significant (p = 0.067). APOE ε4 carriers comprised 38% of the patient group and had higher PiB SUVR in the MCI-LB (p < 0.001), DLB (p = 0.049), and CU (p = 0.006) groups, but not in the iRBD group (p = 0.69; Figure 4).
Figure 3. Predicted 11C-PiB SUVR by Age in the DLB Continuum Sample (N = 168).
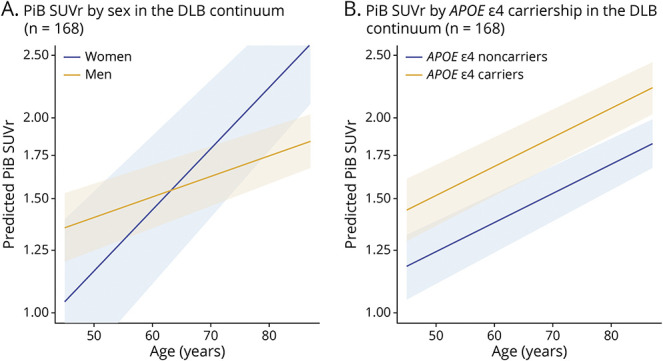
Data were stratified by (A) sex and (B) APOE ε4 carriership. DLB = dementia with Lewy bodies; PiB = Pittsburgh compound B; SUVR = standardized uptake value ratio.
Figure 4. Global Cortical Aβ Accumulation by Sex and APOE ε4 Carriership in the DLB Continuum.
Aβ = β-amyloid; CU = cognitively unimpaired; DLB = dementia with Lewy bodies; iRBD = isolated REM sleep behavior disorder; MCI-LB = mild cognitive impairment with Lewy bodies; PiB = 11C-Pittsburgh compound B; SUVR = standardized uptake value ratio.
Aβ PET Findings and DLB Core Clinical Features
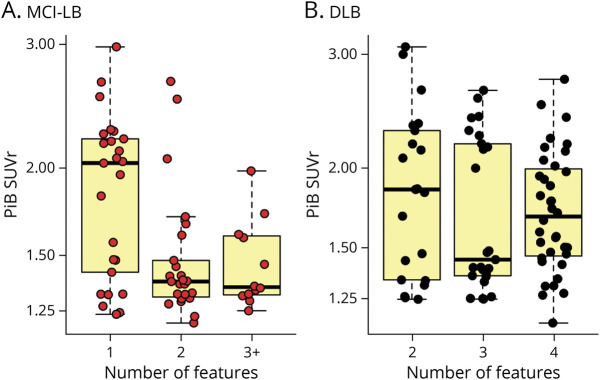
In the MCI-LB group, there was a significant difference in cortical PiB SUVR between patients with 1, 2, and 3 or more DLB clinical features (p = 0.003; Figure 5A). Patients with MCI-LB with 1 core clinical feature of DLB had a higher cortical PiB SUVR than patients with 2 (p = 0.002) and 3 or more (p = 0.021) core clinical features. Among the 25 patients with MCI-LB who had 1 core feature, the breakdown was as follows: RBD (n = 17, 68%), parkinsonism (n = 7, 28%), visual hallucinations (n = 0, 0%), and fluctuations (n = 1, 4%). Patients with MCI-LB with 2 and 3 or more clinical features did not differ in cortical PiB SUVR (p = 0.99). Because indicative biomarkers were not used in the diagnosis of MCI-LB, the certainty (possible vs probable MCI-LB) that MCI is due to Lewy bodies in those with only 1 core clinical feature of DLB is lower than in those with 2 or more clinical features. We thus performed a sensitivity analysis in those with MCI-LB with 1 clinical feature who also have abnormal DaTscan (n = 6). These patients were included in the same group of patients with MCI-LB with 2 or more core clinical features of DLB (n = 45). This group was defined as probable MCI-LB according to current research criteria for MCI-LB.6 Probable MCI-LB did not show significant differences in PiB SUVR compared with CU (p = 0.310; eFigure 1, links.lww.com/WNL/C805). In the DLB group, patients with 2, 3, or 4 clinical features did not show significant differences in PiB SUVR (p = 0.56; Figure 5B).
Figure 5. Global Cortical Aβ Accumulation by the Number of Core Clinical Features.
Differences in global cortical PiB SUVR with increasing number of core clinical features of DLB among patients with (A) MCI-LB and (B) DLB. Aβ = β-amyloid; DLB = dementia with Lewy bodies; MCI-LB = mild cognitive impairment with Lewy bodies; PiB-PET = 11C-Pittsburgh compound B-PET; SUVR = standardized uptake value ratio.
Discussion
In this cross-sectional study, we investigated cortical Aβ deposition on PiB-PET across the DLB continuum, comparing CU with the early prodromal stage of iRBD, the prodromal stage of MCI-LB, and finally DLB. Our findings demonstrate that, in the DLB continuum, elevations of cortical Aβ were observed in MCI-LB and DLB compared with iRBD and CU. Furthermore, PiB SUVR was associated with APOE ε4 carriership along the Lewy body continuum but not with sex. However, when looking at sex differences within each patient subgroup, although the number of women were limited, women with DLB had greater PiB-PET levels than men with DLB. Finally, Aβ in patients with MCI-LB with just 1 core clinical feature tend to have higher levels of cortical Aβ, indicating a higher proportion of patients with AD-related pathology in this group.
In our cohort, increased global cortical PiB SUVR was higher in the DLB and the MCI-LB groups than in controls. In the DLB group, 60% of patients were classified as Aβ positive (PiB SUVR ≥1.48), which is consistent with earlier studies reporting a mean Aβ positivity that ranged from 33% to 69% in DLB.2,3,8-10,29,30 Fewer studies have reported the frequency of Aβ positivity in prodromal DLB than in DLB.5,30 Our data show that up to 41% of patients with MCI-LB are Aβ positive, which is lower in frequency than the observations in patients with DLB. This difference in frequency between MCI-LB and DLB confirms that Aβ may start becoming abnormal at MCI-LB stage, but it continues to accumulate as the disease progresses to DLB, as suggested by previous longitudinal Aβ studies in DLB.31 In the iRBD group, Aβ PET findings were comparable with CU, and only 25% and 19%, respectively, were classified as Aβ positive. An earlier study reported a similar proportion of patients with iRBD who were Aβ PET positive (16.7%), and this rate was significantly lower than DLB (72.7%).32 However, this study did not include a group of CU controls without sleep disorders. Overall, this study provides data showing that accumulation of Aβ in the cortex does not arise in the early prodromal stage of iRBD at a significantly greater frequency than CU adults but increase in Aβ seems to be concomitant with later MCI-LB and DLB stages in the DLB continuum. Whether Aβ accumulation is a precursor to cognitive impairment within the DLB continuum needs further investigation with longitudinal PiB-PET and cognitive testing.
The topographic distribution of elevated cortical Aβ burden in those with DLB compared with controls has been characterized as significantly higher in the frontal, parietal, and superior temporal lobes, with relatively spared occipital and inferior temporal lobes.33,34 We found that, compared with CU, elevated cortical PiB SUVR in MCI-LB and DLB followed a uniform pattern throughout the entire cortex. This discrepancy in the topographic pattern of differences in Aβ burden may be explained by wide variations in PiB retention in a healthy population, which is consistent with previous reports.33,35 Furthermore, there were demographic differences between the controls used in earlier studies and in our study. For example, in Gomperts et al.,34 the group of healthy controls were older and predominantly women, while our CU group were younger on average and predominantly men, similar to the DLB cohort.
Age, female sex, and APOE ε4 carriership have been considered as major risk factors of higher Aβ in DLB.10 In this study, we found that increasing PiB SUVR was indeed associated with age and APOE ε4 genotype with cross-sectional examination of the prodromal and dementia stages of DLB. In agreement with previous results from a large consortium study,10 our findings also indicated that cortical Aβ burden increased in APOE ε4 carriers regardless of age in MCI-LB and DLB groups, suggesting that the influence of APOE ε4 begins even at the prodromal stage of DLB. APOE ε4 may have a direct influence on Aβ accumulation but may also be associated with Lewy-related pathology independent of AD pathology.36 The role of APOE ε4 in DLB and whether it has an indirect influence on clinical phenotypes within the DLB continuum remains to be seen.
Sex was not related to PiB SUVR levels, though low power due to the male predominance in the sample and limited representation of women in our sample may be a factor. The lack of association between PiB SUVR and sex in prodromal DLB is consistent with previous data, which have shown that men and women do not differ in Aβ burden or in the likelihood of Aβ positivity in MCI-LB.5 When plotting sex vs PiB SUVR, we observed that levels of PiB SUVR increased more with age in women than men in the whole DLB continuum. Ferreira et al.10 obtained similar findings in a large cohort of patients with DLB where the frequency of women was significantly higher in those patients classified as Aβ positive according to CSF and PET data. One explanation for this age-dependent sex difference in DLB, as previously reported, is that women are more likely to have a mixed pathology of Lewy body with AD than men.9 Hence, as patients get older and become more impaired, the probability of having elevated amyloid pathology increases, and therefore, the difference between women and men in Aβ accumulation may be more evident. Nonetheless, individuals in our cohort were mostly men throughout the DLB continuum, and therefore, the power to investigate Aβ deposition in women was limited.
Finally, we found an association of Aβ load with the number of core clinical features of DLB in MCI-LB. Following the criteria based on clinical features, patients with DLB can be classified as probable DLB if they have 2 or more clinical features, or possible DLB if only 1 clinical feature is present.12,19 The same scheme is followed in the research criteria recently proposed for MCI-LB.6 We found that the number of DLB core clinical features was associated with PiB SUVR only in the MCI-LB group, but not in DLB. In DLB, previous PET studies have also suggested that Aβ biomarkers do not seem to be associated with the probability of having any of the clinical features of visual hallucinations, cognitive fluctuations, parkinsonism, or RBD.5,10 Our data confirm that Aβ load is not associated with these clinical features in probable DLB. In MCI-LB, the presence of just 1 clinical feature of DLB was associated with a higher global cortical PiB SUVR than having 2 or more clinical features. In other words, the probability of having elevated levels of Aβ pathology was higher in those patients with possible MCI-LB than those with probable MCI-LB. This finding suggests that there might be multiple pathologic pathways under the same diagnostic category of MCI-LB. In a subset of patients with possible MCI-LB with high Aβ, AD pathology may be the dominating etiology of their clinical phenotype, potentially with concomitant Lewy-related pathology. In those with probable MCI-LB, with low Aβ, it is possible that the Lewy body disease may be the dominating etiology. Although this hypothesis needs to be confirmed with neuropathologic and/or longitudinal data, these findings support the construct of the MCI-LB research criteria that possible MCI-LB may have a lower likelihood of progressing to DLB than probable MCI-LB.6
We did not include indicative biomarkers in our analysis because of limited availability of data. It is possible that a subset of patients with MCI-LB with 1 core clinical feature would be distinguished as probable MCI-LB based on indicative biomarker positivity. Despite the limited data on indicative biomarkers of Lewy body disease, we performed a sensitivity analysis in those patients with MCI-LB with 1 core clinical feature of DLB and abnormal DaTscan, a hallmark biomarker of Lewy body disease (n = 6). These patients were added to the group of probable MCI-LB with 2 or more core clinical features of DLB. When those with probable MCI-LB were compared with CU individuals, there were no significant differences in PiB SUVR. This result reinforces the hypothesis that in patients with possible MCI-LB with high Aβ, AD pathology may be the dominating etiology of their clinical phenotype, while in probable MCI-LB, the dominating pathology is Lewy body disease with low Aβ. However, this hypothesis requires further investigation with higher number of patients with MCI-LB and more indicative biomarkers of Lewy body disease.
One limitation of this study is that our data were cross-sectional and did not provide information on longitudinal trajectories of Aβ PET in the DLB continuum or associated risks of clinical progression in iRBD and MCI-LB groups. As expected, participants were mostly men among the 3 clinical groups in the DLB continuum; therefore, the power to investigate sex differences in Aβ PET was limited. Qualitatively, women tend to have higher levels of Aβ PET than men within each group, but this difference was significant only in the DLB group with highest Aβ levels. However, we found that women tend to accumulate more Aβ as they get older than men. Therefore, both disease severity and age seem to be influential in Aβ accumulation. More studies with balanced number of men and women are still needed to fully understand sex differences in Aβ PET within the DLB continuum. Furthermore, it is known that other biomarkers, such as striatal dopamine transporter availability, brain glucose metabolism, or tau pathology are also associated with different clinical and pathologic aspects of the disease in iRBD, MCI-LB, and DLB. We hypothesize that amyloid PET and other biomarkers will be complementary in characterizing clinical progression and trajectories in patients with MCI-LB and DLB, as suggested before in a previous study.5 Abnormal tau burden on PET will co-occur with high amyloid load on PET in DLB and perhaps in a lower proportion in MCI-LB. We also hypothesize that longitudinal change in these biomarkers will be associated with longitudinal cognitive decline in domains primarily involved in DLB and that these associations will allow to differentiate between DLB and AD at prodromal stages of the diseases. Finally, age, sex, and APOE ε4 will keep modulating these outcomes. Further investigations with longitudinal designs and multiple imaging biomarkers are still needed to test these hypotheses.
Current data demonstrate that, in the DLB continuum, abnormal cortical Aβ accumulation in the cortex occurs along with cognitive impairment, being significantly higher at MCI-LB and DLB stages, but not at iRBD. Furthermore, APOE e4 carriership is an important contributor to higher Aβ levels at both MCI-LB and DLB stages. Finally, Aβ load is associated with the number of core clinical features of DLB that differentiate possible and probable categories in MCI-LB. Patients with possible MCI-LB with just 1 core clinical feature of DLB tend to have more cortical Aβ pathology than probable MCI-LB. Therefore, possible MCI-LB with high Aβ pathology may potentially evolve into an AD phenotype with Lewy body disease as a contributing etiology of cognitive impairment. Indicative biomarkers of DLB may further distinguish the possible MCI-LB who can potentially follow the DLB vs AD dementia pathways. These findings have important implications for future clinical trials targeting patients within the DLB continuum during the prodromal stage.
Acknowledgment
The authors thank the patients and their family members for participating in this research.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ANCOVA
analysis of covariance
- CDR
Clinical Dementia Rating
- CDR-SOB
CDR Sum of Boxes
- CU
cognitively unimpaired
- DaTscan
123I-FP-CIT SPECT
- DLB
dementia with Lewy bodies
- FDR
false discovery rate
- iRBD
isolated REM sleep behavior disorder
- MCALT
Mayo Clinic Adult Lifespan Template
- MCI-LB
mild cognitive impairment with Lewy bodies
- MCSA
Mayo Clinic Study of Aging
- MMSE
Mini-Mental State Examination
- PiB
Pittsburgh compound B
- PSG
polysomnography
- SPM
statistical parametric mapping
- SUVR
standardized uptake value ratio
Appendix. Authors
Study Funding
This work is supported by the NIH grants U01 NS100620, P50 AG016574, P30 AG 062677, R34 AG056639, and U01 AG006786; Foundation Dr. Corinne Schulerand; the Mangurian Foundation for Lewy Body Research; The Elsie and Marvin Dekelboum Family Foundation; GHR; Mayo Medical Foundation for Education and Research; Little Family Foundation; and LBD Functional Genomics Program.
Disclosure
The authors report no disclosure relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Ferman TJ, Aoki N, Crook JE, et al. The limbic and neocortical contribution of α-synuclein, tau, and amyloid β to disease duration in dementia with Lewy bodies. Alzheimers Dement. 2018;14(3):330-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker L, McAleese KE, Thomas AJ, et al. Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015;129(5):729-748. [DOI] [PubMed] [Google Scholar]
- 4.Bousiges O, Cretin B, Lavaux T, et al. Diagnostic value of cerebrospinal fluid biomarkers (phospho-tau181, total-tau, Aβ42, and Aβ40) in prodromal stage of Alzheimer's disease and dementia with Lewy bodies. J Alzheimers Dis. 2016;51(4):1069-1083. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Lowe VJ, Boeve BF, et al. β-Amyloid PET and (123)I-FP-CIT SPECT in mild cognitive impairment at risk for Lewy body dementia. Neurology. 2021;96(8):e1180-e1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeith IG, Ferman TJ, Thomas AJ, et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94(17):743-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Postuma RB, Iranzo A, Hu M, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019;142(3):744-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313(19):1939-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarci K, Lowe VJ, Chen Q, et al. β-Amyloid PET and neuropathology in dementia with Lewy bodies. Neurology. 2020;94(3):e282-e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira D, Przybelski SA, Lesnick TG, et al. β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology. 2020;95(24):e3257-e3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia C, Dickerson BC. Multimodal PET imaging of amyloid and tau pathology in Alzheimer disease and non-Alzheimer disease dementias. PET Clin. 2017;12(3):351-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas AJ, Taylor JP, McKeith I, et al. Revision of assessment toolkits for improving the diagnosis of Lewy body dementia: the DIAMOND Lewy study. Int J Geriatr Psychiatry. 2018;33(10):1293-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galvin JE. Improving the clinical detection of Lewy body dementia with the Lewy body composite risk score. Alzheimers Dement (Amst). 2015;1(3):316-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belden CM, Kahlon V, Malek-Ahmadi M, Tsai A, Sabbagh MN. Clinical characterization of mild cognitive impairment as a prodrome to dementia with Lewy bodies. Am J Alzheimers Dis Other Demen. 2015;30(2):173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Génier Marchand D, Postuma RB, Escudier F, et al. How does dementia with Lewy bodies start? prodromal cognitive changes in REM sleep behavior disorder. Ann Neurol. 2018;83(5):1016-1026. [DOI] [PubMed] [Google Scholar]
- 17.Donaghy PC, Taylor JP, O'Brien JT, et al. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol Med. 2018;48(14):2384-2390. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Sleep Medicine. International Classification of Sleep Disorders-2: Diagnostic and Coding Manual. AASM; 2005. [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. [DOI] [PubMed] [Google Scholar]
- 21.Ivnik RJ, Malec JF, Smith GE, et al. Mayo's older Americans normative studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychologist. 1992;6:1-104. [Google Scholar]
- 22.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140(6):566-572. [DOI] [PubMed] [Google Scholar]
- 25.Lowe VJ, Lundt ES, Senjem ML, et al. White matter reference region in PET studies of (11)C-Pittsburgh compound B uptake: effects of age and amyloid-β deposition. J Nucl Med. 2018;59(10):1583-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwarz CG, Gunter JL, Ward CP, et al. [P2–415]: the Mayo Clinic Adult Lifespan Template: better quantification across the lifespan. Alzheimers Dement. 2017;13(7S pt 16):P792. [Google Scholar]
- 27.Jack CR Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13(3):205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maltais DD, Jordan LG, Min HK, et al. Confirmation of (123)I-FP-CIT SPECT quantification methods in dementia with Lewy bodies and other neurodegenerative disorders. J Nucl Med. 2020;61(11):1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donaghy PC, Firbank MJ, Thomas AJ, et al. Amyloid imaging and longitudinal clinical progression in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2020;28(5):573-577. [DOI] [PubMed] [Google Scholar]
- 30.Yoo HS, Lee S, Chung SJ, et al. Dopaminergic depletion, β-amyloid burden, and cognition in Lewy body disease. Ann Neurol. 2020;87(5):739-750. [DOI] [PubMed] [Google Scholar]
- 31.Nedelska Z, Schwarz CG, Lesnick TG, et al. Association of longitudinal β-amyloid accumulation determined by positron emission tomography with clinical and cognitive decline in adults with probable Lewy body dementia. JAMA Netw Open. 2019;2(12):e1916439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi R, Hayashi H, Kawakatsu S, Okamura N, Yoshioka M, Otani K. Assessment of amyloid deposition in patients with probable REM sleep behavior disorder as a prodromal symptom of dementia with Lewy bodies using PiB-PET. Front Neurol. 2019;10:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomperts SN, Locascio JJ, Marquie M, et al. Brain amyloid and cognition in Lewy body diseases. Mov Disord. 2012;27(8):965-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson DW, Heckman MG, Murray ME, et al. APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology. 2018;91(12):e1182-e1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study are available from the corresponding author on reasonable request.