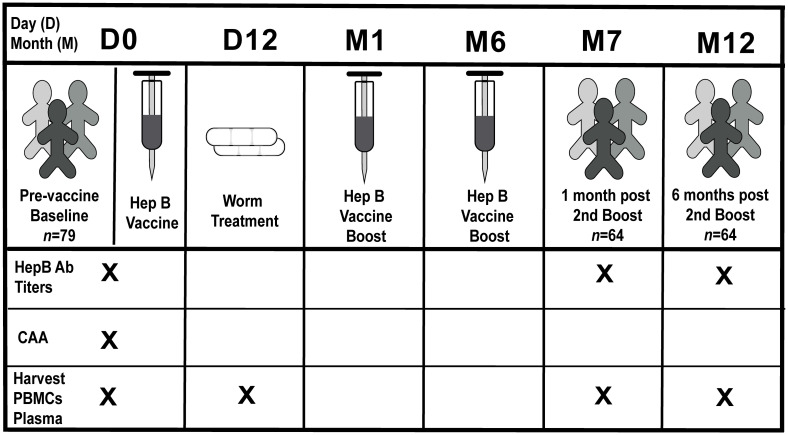
Fig 1. Clinical study design.
Participants enrolled in the clinical study (n = 79, four donor samples were unavailable for analysis in this study) were vaccinated at baseline [(pre-vaccination) day 0 (D0)] and received two boosters at month 1 (M1) and month 6 (M6). Sera samples were collected at D0, month 7 post-vaccination (M7) (one month post-booster 2) and month 12 post-vaccination (M12) (six months post-booster 2), and PMBCs and plasma were collected at D0, D12, M7 and M12 for subsequent analysis. Stool samples were collected at D0, D3 or D7 for egg count analysis. Egg positive individuals were treated at day 12 post-vaccination (D12) with Praziquantel (PZQ). X denotes corresponding samples collected at that timepoint.