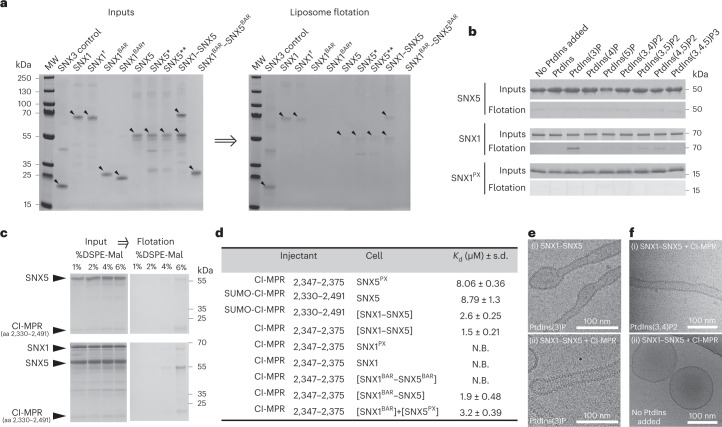
Fig. 2. Membrane recruitment and coat organization is influenced by dimerization, phosphoinositides and cooperative interactions with cargo.
a, Effects of SNX1 and SNX5 interface mutations on the association with liposomes (DOPC:DOPE:DOPS:PtdIns(3)P:Liss Rhod PE in a 45:28:20:5:2 molar ratio) by flotation assay. MW, molecular weight marker. Note that association with the membrane was enhanced by dimerization of full-length SNXs but not by heterodimers of BAR domains alone. All SDS–PAGE samples that originated from flotation assays were normalized relative to their Liss Rhod PE content. b, Liposome flotation analyses to characterize the binding of SNX5, SNX1 and SNX1PX to specific phosphoinositides. Note that only full-length SNX1 interacts specifically with PtdIns(3)P, and to a minor extent with PtdIns(4,5)P2, PtdIns(3,5)P2 and PtdIns(3,4)P2. c, CI-MPR promotes membrane recruitment of SNX5, and this effect is enhanced in the presence of SNX1. Flotation assay of liposomes functionalized with the cytosolic tail of CI-MPR. CI-MPR was conjugated with increasing concentrations of DSPE-Mal on the surface of liposomes containing no phosphoinositides to exclude their specific interaction with SNX1. aa, amino acids. d, SNX1BAR domain enhances the interaction between the PX domain of SNX5 and CI-MPR. Summary of Kd values between CI-MPR and SNX1–SNX5 or various subdomains from the heterodimer. Values are the mean ± s.d. from at least two independent experiments. N.B. no binding. e, Representative cryo-transmission electron microscopy (cryo-TEM) images of liposomes (DOPC:DOPE:DOPS:PtdIns(3)P in a 45:30:20:5 molar ratio) incubated with SNX1–SNX5 in the absence (i) or presence (ii) of the cytoplasmic tail of CI-MPR. f, Representative cryo-TEM images of liposomes incubated with SNX1–SNX5 and the cytoplasmic tail of CI-MPR in the presence of PtdIns(3,4)P2 (i) or in the absence of PtdIns (ii). Data are representative of three (a–c) or two (e, f) independent experiments.