Key Clinical Message
Medical records of a 76‐year‐old woman with a recurrent acinic cell carcinoma of the left parotid gland with lateral skull base invasion were reviewed. She underwent subtotal petrosectomy followed by radiation therapy. After surgery, she remained disease‐free for more than 16 months.
Keywords: acinic cell carcinoma, lateral skull base, parotid gland, salivary glands
1. INTRODUCTION
Acinic cell carcinoma (AciCC) is an uncommon malignant epithelial neoplasm of the salivary glands typically arising in the parotid gland (85% of cases) 1 , 2 ; it comprises 7%–15% of all malignant tumors of the major salivary glands, and 5% of those of parotid gland. 3 , 4
At first classified as a “benign adenoma” (or “acinic cell tumor”) by Goodwin et al. 5 in 1890, Buxton et al. 6 recognized it as a malignant tumor in 1953, and recently WHO re‐classified AciCC as malignant carcinoma. 7
Women are affected only slightly more frequently than men, with a slight peak in the fifth and sixth decades; patients ranging from young children to centenarians. 8 Possible causes of AciCC include previous radiation exposure and familiar predisposition. There are no distinctive radiological patterns (MRI, CT, ultrasound imaging), and it commonly occurs as an indolent, well‐defined solid cystic mass.
Complete surgical excision of the primary tumor offers the best opportunity for cure. 9 Radiation therapy should be considered in some selected cases (positive surgical margins, multiple positive lymph nodal involvement, perineural invasion, high histologic grade, stage T3 or T4). However, the role of radiotherapy and chemotherapy remains unclear. 10 To the best of our knowledge, descriptions of parotid AciCC with skull base invasion have been limited only to 12 case reports. 11 , 12 , 13
The aim of this paper is to report a case of recurrent AciCC of the parotid gland with lateral skull base invasion and to review the existing literature about this rare neoplasm.
2. CASE REPORT
A 74‐year‐old woman was admitted in June 2018 to our Otolaryngology Department with a hard, indolent and fixed left parotid mass. The patient reported onset of symptoms 3 months earlier. Physical examination showed no facial paralysis. Ten years before she had a left breast cancer and actually she took no medications. She was not an active smoker with no alcohol or drug use.
A CT of the neck after contrast showed a 2 × 3 × 3 cm heterogeneously enhanced mass in the deep lobe of the left parotid gland, without extraparotid extension. No lymph node abnormalities were observed.
An ultrasound guided fine needle aspiration biopsy (FNAB) was performed and the citology suggested a poorly differentiated basal cells neoplasm (cT2N0M0). Consequently, the Head and Neck Tumor Board recommended a total surgical excision.
The patient underwent a total left parotidectomy with facial nerve monitoring and facial nerve preservation. She was discharged 5 days after surgery and recovered without incident. A final pathology showed an AciCC of the left parotid gland with low degree of malignancy, without perineural invasion and with free resection margins (pT3N0M0).
As a result, the Head and Neck Tumor Board, supported by the last medical literature, did not recommend complementary radiation therapy.
She reported at the Head and Neck Department in February 2019, due to otalgia and otorrhea from the left ear and complete facial paralysis; the physical examination showed a swelling at the site of the previous surgery.
A CT of the neck with intravenous contrast, showed many colliquative areas in the parotid area, with lytic tissue extended to the external auditory canal, the tympanic cavity, and to the mastoid, with infiltration of the sigmoid sinus and erosion of the labyrinthine block up to the internal auditory canal and to the pontocerebellar angle (Figure 1).
FIGURE 1.
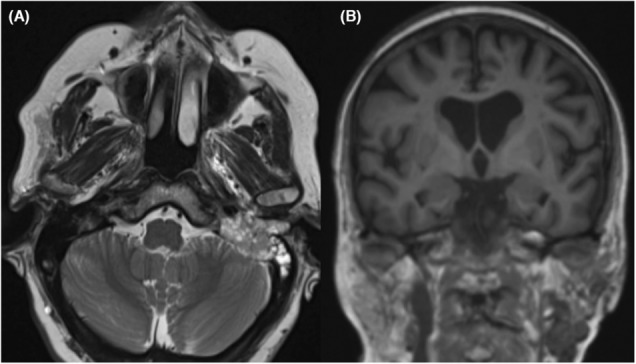
Pre‐operative CT Scans; (A) Axial CT Section; the tumor involved the left pontocerebellar angle; (B) Coronal CT Section; the neoplasm involved the left petrous bone, the left pontocerebellar angle and the fibers of VII, VIII, IX, X, XI, XII cranial nerves.
A FNAB was performed and citology revealed a recurrence of AciCC of the parotid gland.
The patient underwent a lateral skull base resection. A lateral approach to the skull base by pre‐sigmoid‐retrolabirinthine has been performed. The posterior and lateral semicircular canals have been skeletonized. The facial nerve was exposed in its second genu and the rest of the nerve was encased by the tumor; therefore, it was not possible to preserve it. The jugular bulb, very thin, started bleeding during the delicate dissection of the tumor, and bleeding was stopped by using Gelfoam. By introducing the endoscope, the clivus and the abducens nerve were on the view. A total excision of the tumor up to the left pontocerebellar angle 14 , 15 and a left modified neck radical dissection (levels I–V) with external carotid artery ligation were performed. Surgery was performed by using microscope and endoscope, extremely useful in order to check all the corners of the tumor bed, to be sure that a total removal had been performed. The defect of the dura mater was repaired by a dura patch, and the petromastoid cavity was filled by using abdominal fat and fibrin glue, in order to avoid cerebrospinal fluid distulas. Spinal drainage was not inserted.
The histologic examination was in favor of a recurrence of AciCC (Figure 2) (pT4N1M0).
FIGURE 2.
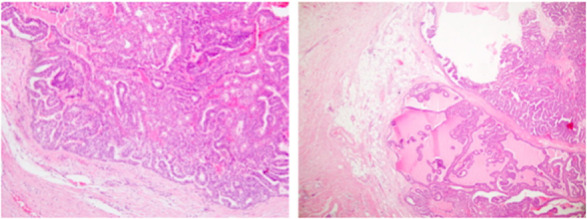
Post‐operative hystologic examination showed an acinic cell carcinoma.
Postoperative outcome was good and she was discharged from hospital 2 weeks after surgery, with recommendation to start treatment with neutron radiotherapy at a total dose of 66 Gy, without chemotherapy. A CT scan, performed 3 months and 6 months after radiation therapy, showed stable disease.
At a recent follow‐up, 10 days ago, more than 16 months after surgical revision, an MRI with contrast showed, in the site of previous left petrosectomy, an heterogeneously enhanced tissue growing through the foramen lacerum and involving the foramen magnum (Figure 3). Therefore, the MRI scan showed a non‐enhancing ovalar mass (2 × 2 cm) in the left thyrohyoid membrane, and left retroauricolar lymph nodes with coagulative necrosis (Figure 4). We suggested a decompressive surgery, but the patient refused surgery and decided for hospice care.
FIGURE 3.
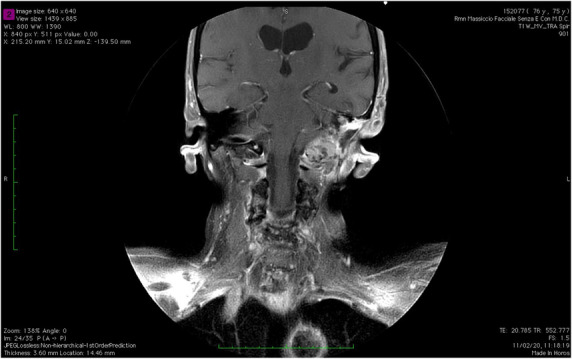
Sixteen months MRI follow up (coronal view). In the site of previous left petrosectomy the MRI scan showed an heterogeneously enhanced tissue; toward the rear, it grows through the foramen lacerum involving the foramen magnum.
FIGURE 4.
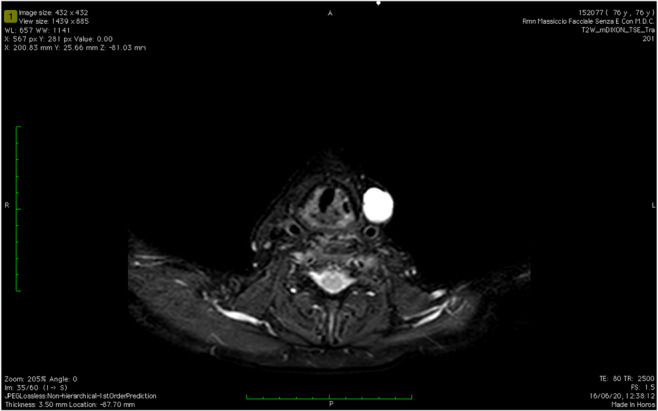
Sixteen months MRI follow up (axial view). Non‐enhancing ovalar mass (2 × 2 cm) in the left thyrohyoid membrane.
3. DISCUSSION
Considered benign for up to a few decades, AciCC of the parotid gland has recently been classified as a malignant tumor. Due to its rarity, AciCC has received less attention than other salivary neoplasms.
Diagnosis of AciCC is frequently complicated, due to its similarity with benign tumors. The differential diagnosis is considered, fundamentally, with clear cell carcinomas, mucoepidermoid carcinomas, Warthin's tumor, and oncocytomas. The greatest diagnostic challenge is to differenziate ACC from mammary analogue secretory carcinoma (MASC), a new entity among salivary glands malignancy introduced in the 4th edition of the WHO classification of head and neck tumors. 16
There are no clear characteristics of AciCCs found on CT, MRI, and ultrasound imaging.
Management of AciCC, especially when metastatic, is very challenging: because of the rarity, data on management are based only on personal clinical experience and retrospective series. The preferred treatment of local disease is complete surgical excision, consisting in total parotidectomy.
The role of radiotherapy remains controversial: the precise indications and oncologic effects of adjuvant radiotherapy in AciCC of the parotid gland are not well known, particularly in patients with negative, but close (<1 mm) margins without other high‐risk histopathologic factors.
According to the most recent literature, the main factors that may predict poor outcomes are (1) extracapsular extension, (2) facial nerve involvement, (3) positive surgical margins, (4) cellular atypia, (5) high pathologic grade as evidenced by >2 mitoses/10 high power fields, (6) necrosis, (7) perineural and vascular invasion, (8) desmoplastic stromal reaction. To our knowledge, only 12 cases of AciCC of the parotid gland with lateral skull base involvement have been reported in literature.
Breen JT et al. 11 described a unique series of 10 consecutive patients with primary or recurrent AciCC of the parotid gland with skull base invasion or metastasis. After developing skull base disease, four patients underwent surgery, four stereotactic radiosurgery and three external beam radiation. The 10‐year Kaplan–Meyer estimated overall survival after initial diagnosis of parotid AciCC was 80%; after lateral skull base invasion, 2‐year estimated overall survival was 50%.
Buiret G et al. 12 reported a case of a 62‐year‐old woman with AciCC and skull base invasion treated by external 3D conformational radiation. The tumor showed a regular regression over 2.5‐year period without side‐effects of radiation. They suggested that exclusive external radiation therapy could be an option in case of contra‐indication for surgery or patient refusal.
A study by Douglas JG et al. 13 suggested that patients with salivary neoplasms with lateral skull base invasion should be considered for a Gamma knife boost after primary treatment with neutron radiotherapy; however, among the patients considered by the study, only one has AciCC with skull base involvement.
The patients described had the following characteristics (we previously excluded the patient from the study of Douglas JG et al., because the authors did not report any data) (Table I); men to women patient ratio was approximately 1:1. Median age was 52.9 years (range, 18–79), and only two patients (18.2%) had skull base disease at initial presentation. Six patients (54.5%) underwent total parotidectomy while three (27.2%) had superficial parotidectomy; in other two patients (18.2%), it is not reported the type of initial surgery. After surgery, only two patients (18.2%) showed positive excision margins; therefore, two patients (18.2%) underwent adjuvant radiotherapy.
TABLE I.
Characteristics of the patients reported in the studies included in the review.
| Study | Patient, gender, age at diagnosis (years) | Surgical approach) | Margins at initial excision | Initial adjuvant therapy | Skull base involvement at initial diagnosis | Time to development of skull base disease (years) and localization | Skull base disease treatment | Positive cervical lymph nodes / Distant metastases |
|---|---|---|---|---|---|---|---|---|
| Breen JT et al. 11 | 1, F, 18 years | Total parotidectomy | R1 | None | No | 12; Glenoid fossa, infratemporal fossa, Foramen ovale, Meckel's cave, cavernous sinus, sphenoid sinus, clivus, cerebellum | Extended parotidectomy, partial mandibulectomy, fibular free flap (FFF), external beam and stereotactic radiation | − / + |
| 2, M, 76 years | Superficial parotidectomy | DNA | None | No | 10.1; Masticator space and temporal fossa | External beam radiation | + / − | |
| 3, F, 23 years | Superficial parotidectomy | R0 | None | No | 47.2; Parapharyngeal space and petrous temporal bone | LTBR, partial mandibulectomy, FFF, developed subsequent recurrence at petrous temporale bone and clivus | − / + | |
| 4, M, 49 years | Superficial parotidectomy | R0 | None | No | 14.3; stylomastoid foramen, EAC | Revision parotidectomy, LTBR, external auditory canal resection, SCM flap. | + / − | |
| 5, F, 79 years | Total parotidectomy | R0 | RT | No | 4; stylomastoid foramen and jugular foramen | None | − / − | |
| 6, M, 55 years | Total parotidectomy | R0 | RT | Yes | DNA; EAC, petrous temporal bone and mastoid, posterior cranial fossa | Subtotal petrosectomy, removal of sigmoid sinus and adjacent dura, trapezius flap, stereotactic radiation | − / + | |
| 7, M, 61 years | None | DNA | DNA | Yes | DNA; On original presentation —TMJ, parapharyngeal space, foramen ovale | None | + / + | |
| 8, M, 72 years | Total parotidectomy | R0 | None | No | 10.3; Sphenoid sinus, cavernous sinus, clivus, parietal bone | Stereotactic radiation | − / − | |
| 9, F, 39 years | Parotidectomy of unknown extent | DNA | None | No | 36.5; stylomastoid foramen | None | − / − | |
| 10, F, 48 years | Total parotidetomy | R0 | None | No | 15.3; stylomastoid foramen | Stereotactic radiation | − / − | |
| Buiret G et al. 12 | 11, F, 62 years | Total parotidectomy | R0 | None | No | 8; inferior side of the temporal bone with conserved inner and middle ear and brain structures ad no meningeal involvement | Stereotactic radiation, revision surgery | + / − |
| Douglas JG et al. 13 | 12, DNA, DNA | DNA | DNA | DNA | DNA | DNA, DNA | Gamma knife boost | DNA |
The mean time to develop lateral skull base disease was 15.8 years (range, 4–47.2) (of two patients are not reported these data). This recurrence was treated by surgery alone in two cases (18.2%), with radiotherapy alone in three cases (27.3%), and with revision surgery followed by radiation therapy in three cases (27.3%); for three patients (27.3%) this item is not specified. No patient was treated with chemotherapy regime, confirming the lack of utility of chemotherapy against this histological type of parotid cancer.
Four patients (36.4%) had distant metastases, while other four (36.4%) had cervical metastases. Most frequent skull base localizations were: stylomastoid foramen (4 patients, 36.4%), petrous temporal bone (3 patients, 27.3%), clivus (three patients, 27.3%), foramen ovale (2 patients, 18.2%), cavernous sinus (2 patients, 18.2%), sphenoid sinus (2 patients, 18.2%).
4. CONCLUSION
Knowledge about AciCC has changed over the past few decades. Surgery is the first‐line treatment, while radiotherapy can be helpful in selected patients (as patients with positive surgical margins) and in case of recurrence. No chemotherapy regimen is currently being validated.
From the literature, it emerges that a small number of patients will develop lateral skull base involvement, often years after the treatment of the primary disease. These subjects with cranial base disease are at a high risk for multiple late recurrences and even death.
Although in literature there are no clear evidences about neurotrophicity of AciCC of the parotid gland, in our opinion, very rarely these tumors can grow up through the cranial nerves sheath developing within the posterior cranial fossa.
AUTHOR CONTRIBUTIONS
Pietro De Luca: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; resources; validation; visualization; writing – original draft. Matteo Calvanese: Data curation; formal analysis; writing – original draft. Angelo Camaioni: Formal analysis; supervision; validation; writing – original draft. Giorgio Iaconetta: Formal analysis; supervision; validation; writing – original draft. Maurizio Iemma: Conceptualization; data curation; formal analysis; supervision; validation; writing – original draft.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to disclose. No fundings to declare.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
De Luca P, Calvanese M, Camaioni A, Iaconetta G, Iemma M. Recurrent acinic cell carcinoma of the parotid gland with lateral skull base invasion: Case report and discussion of the literature. Clin Case Rep. 2023;11:e7512. doi: 10.1002/ccr3.7512
REFERENCES
- 1. Kjelfa Y, Mansour M, Abdel‐Aziz Y, et al. Relapsed acinic cell carcinoma of the parotid gland with diffuse distant metastasis: case report with literature review. Med High Impact Case Rep. 2016;4(4):2324709616674742. doi: 10.1177/2324709616674742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Luca P, Tassone D, de Campora L, et al. Cribriform adenocarcinoma of the tongue and minor salivary glands: a systematic review of an uncommon clinicopathological entity. Eur Arch Otorhinolaryngol. 2022;279(6):2719‐2725. doi: 10.1007/s00405-021-07140-6 [DOI] [PubMed] [Google Scholar]
- 3. Son E, Panwar A, Mosher CG, et al. Cancers of the major salivary gland. J Oncol Pract. 2018;14(2):99‐108. doi: 10.1200/JOP.2017.026856 [DOI] [PubMed] [Google Scholar]
- 4. De Luca P, de Campora L, Tassone D, et al. Acinic cell carcinoma of the parotid gland: Timeo Danaos et dona ferentes? A multicenter retrospective analysis focusing on survival outcome. Eur Arch Otorhinolaryngol. 2022;279(12):5821‐5829. doi: 10.1007/s00405-022-07481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Godwin JT, Foote FW Jr, Frazell EL. Acinic cell adenocarcinoma of the parotid gland; report of twenty‐seven cases. Am J Pathol. 1954;30:465‐477. Epub 2022 [PMC free article] [PubMed] [Google Scholar]
- 6. Buxton RW, Maxwell JH, French AJ. Surgical treatment of epithelial tumors of the parotid gland. Surg Gynecol Obstet. 1953;97(4):401‐416. [PubMed] [Google Scholar]
- 7. Al Zaher N, Obeid A, Al‐Salam S, et al. Acinic cell carcinoma of the salivary glands: a literature review. Hematol Oncol Stem Cel Ther. 2009;2(1):259‐264. doi: 10.1016/s1658-3876(09)50035-0 [DOI] [PubMed] [Google Scholar]
- 8. Greig SR, Chaplin JM, McIvor NP, et al. Acinic cell carcinoma of the parotid gland: Auckland experience and literature review. ANZ J Surg. 2008;78(9):754‐758. doi: 10.1111/j.1445-2197.2008.04643.x [DOI] [PubMed] [Google Scholar]
- 9. Cavaliere M, De Luca P, Scarpa A, et al. Acinic cell carcinoma of the parotid gland: from pathogenesis to management: a review. Eur Arch Otorhinolaryngol. 2020;277:2673‐2679. doi: 10.1007/s00405-020-05998-6 [DOI] [PubMed] [Google Scholar]
- 10. Andreoli MT, Andreoli SM, Shrime MG, Devaiah AK. Radiotherapy in parotid acinic cell carcinoma: does it have an impact on survival? Arch Otolaryngol Head Neck Surg. 2012;138(5):463‐466. doi: 10.1001/archoto.2012.226 [DOI] [PubMed] [Google Scholar]
- 11. Breen JT, Carlson ML, Link MJ, Moore E, Neff B, Driscoll C. Skull base involvement by acinic carcinoma of the parotid gland. J Neurol Surg B Skull Base. 2012;73(6):371‐378. doi: 10.1055/s-0032-1322797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buiret G, Céruse P, Ramade A, Carrie C, Pommier P. Acinic cell carcinoma of the parotid gland with skull base invasion: case study, managed by exclusive external 3D radiation therapy. Eur Ann Otorhinolaryngol Head Neck Dis. 2012;129(2):111‐114. doi: 10.1016/j.anorl.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 13. Douglas JG, Goodkin R, Laramore GE. Gamma knife stereotactic radiosurgery for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008;30(4):492‐496. doi: 10.1002/hed.20729 [DOI] [PubMed] [Google Scholar]
- 14. Iaconetta G, Fusco M, Samii M. The Sphenopetroclival venous gulf: a microanatomical study. J Neurosurg. 2003;99(2):366‐375. doi: 10.3171/jns.2003.99.2.0366 [DOI] [PubMed] [Google Scholar]
- 15. Iaconetta G, Fusco M, Cavallo LM, Cappabianca P, Samii M, Tschabitscher M. The abducens nerve: microanatomic and endoscopic study. Neurosurgey. 2007;61(3 Suppl):7‐14. doi: 10.1227/01.neu.0000289706.42061.19 [DOI] [PubMed] [Google Scholar]
- 16. El‐Naggar AK, Chan LKC, Grandis JR, et al. WHO classification of head and neck tumours. WHO Classification of Head and Neck Tumors, Fourth Edition. Vol 9; 2017. [Google Scholar]


