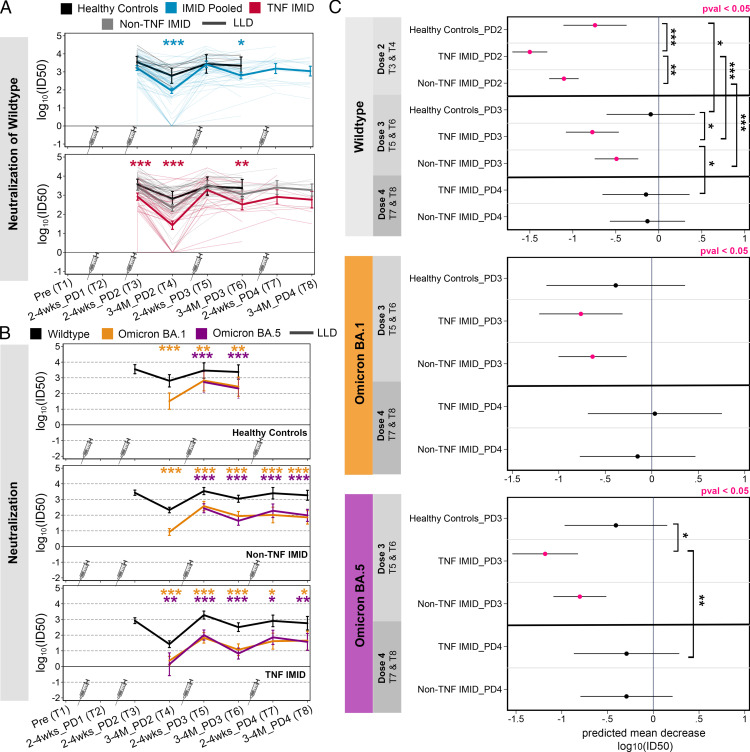
FIGURE 4.
Effect of third and fourth vaccine doses on neutralization activity against WT SARS-CoV-2 and VOCs. Data are reported as a longitudinal analysis of linked samples for the neutralization responses after two to four doses of SARS-CoV-2 mRNA vaccine. Time points are defined in Table I. (A) Predicted neutralization [log10(ID50), NT: neutralization] activity against WT SARS-CoV-2 after two to four doses of vaccine, grouped by healthy control subjects (black), patients with IMIDs pooled (blue), patients with IMID treated with anti-TNF or anti-TNF+MTX/AZA (red), and patients with IMID not treated with anti-TNF (gray). Comparisons between groups are indicated: IMID pooled versus healthy control subjects (blue asterisks) or TNF IMID versus IMID pooled (red asterisks). (B) Predicted neutralization [log10(ID50)] activity against WT, Omicron BA.1, and Omicron BA.5 after two to four doses of vaccine, grouped by healthy control subjects, patients with IMIDs not treated with anti-TNF, and patients with IMID treated with anti-TNF or anti-TNF+MTX/AZA. Neutralization activity against WT is depicted in black, against Omicron BA.1 in orange, and against Omicron BA.5 in purple. Paired t tests were conducted to compare responses to Omicron BA.1 or BA.5 versus WT. (C) Predicted mean decrease of neutralization against WT, Omicron BA.1 and BA.5 in healthy control subjects, TNF IMID, and patients with IMIDs not treated with anti-TNF (between 2–4 wk and 3–4 mo) after dose 2 (decrease between T3 and T4), after dose 3 (decrease between T5 and T6), and after dose 4 (decrease between T7 and T8). Mean decrease values that are significant (p < 0.05) are colored in pink. Significant pairwise comparisons are indicated by asterisks and brackets. (A, C) Sample sizes are listed in Supplemental Table I. (B) Sample sizes are as follows: [WT versus BA.1] n = 9 for HC/T4; n = 5 for HC/T5; n = 6 for HC/T6; n = 48 for non-TNF/T4; n = 24 for non-TNF/T5; n = 25 for non-TNF/T6; n = 8 for non-TNF/T7; n = 11 for non-TNF/T8; n = 32 for TNF/T4; n = 19 for TNF/T5; n = 15 for TNF/T6; n = 9 for TNF/T7; n = 6 for TNF/T8; [WT versus BA.5] n = 5 for HC/T5; n = 6 for HC/T6; n = 24 for non-TNF/T5; n = 25 for non-TNF/T6; n = 8 for non-TNF/T7; n = 11 for non-TNF/T8; n = 4 for TNF/T4; n = 19 for TNF/T5; n = 15 for TNF/T6; n = 9 for TNF/T7; n = 6 for TNF/T8. (A–C) Mixed-effects linear regression models controlled for age, BMI, sex, and vaccine type, with an interaction term between time point and study group. *p < 0.05, **p < 0.01, ***p < 0.001. BAU/ml, binding Ab units per milliliter; HC, healthy control subjects; LLD, lower limit of detection; log10(ID50), serum dilution that inhibits 50% of lentiviral infection; PD, postdose.