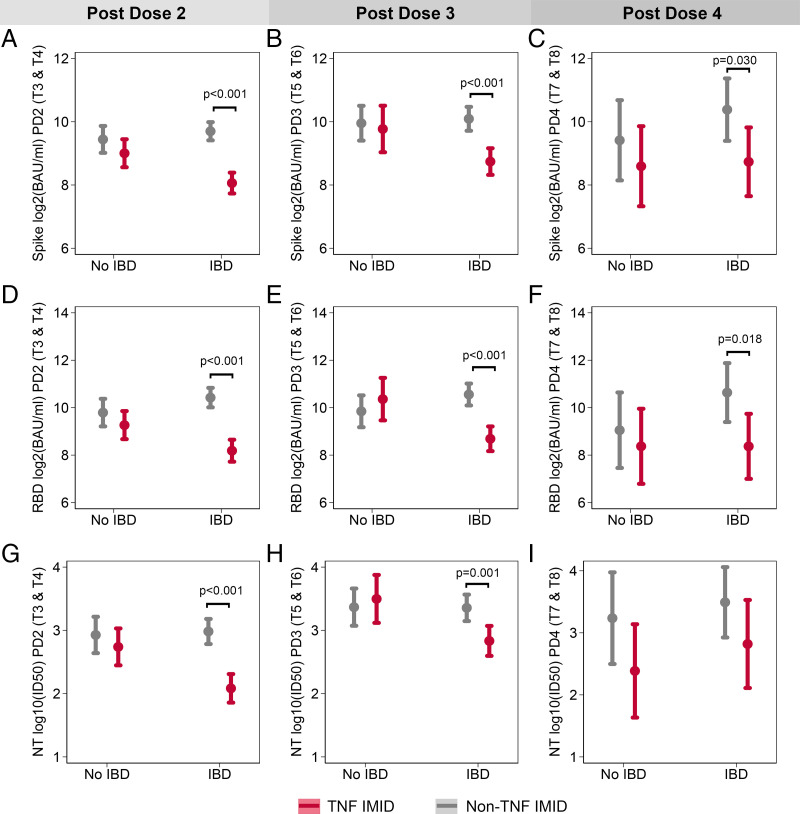
FIGURE 5.
The effect of anti-TNF therapy in reducing Ab responses is significant only in the IBD patient group. The effect of anti-TNF therapy on anti-S IgG (A–C), anti-RBD IgG (D–F), and neutralization of WT SARS-CoV-2 (G–I) was tested for participants with and without IBD using mixed-effects models with an interaction term between IBD status and treatment with anti-TNF therapy. Models controlled for days after vaccination, sex, age, BMI, and vaccine type and were repeated for postdose 2 (A, D, G), postdose 3 (B, E, H), and postdose 4 (C, F, I) time points. For IgG data, samples sizes for each subgroup were as follows: n = 38 for non-IBD/non-TNF/PD2, n = 28 for non-IBD/TNF/PD2, n = 68 for IBD/non-TNF/PD2, n = 58 for IBD/TNF/PD2, n = 21 for non-IBD/non-TNF/PD3, n = 9 for non-IBD/TNF/PD3, n = 34 for IBD/non-TNF/PD3, n = 29 for IBD/TNF/PD3, n = 10 for non-IBD/non-TNF/PD4, n = 9 for non-IBD/TNF/PD4, n = 44 IBD/non-TNF/PD4, and n = 11 for IBD/TNF/PD4. For neutralization data, samples sizes for each subgroup were as follows: n = 36 for non-IBD/non-TNF/PD2, n = 28 for non-IBD/TNF/PD2, n = 67 for IBD/non-TNF/PD2, n = 51 for IBD/TNF/PD2, n = 20 for non-IBD/non-TNF/PD3, n = 9 for non-IBD/TNF/PD3, n = 29 for IBD/non-TNF/PD3, n = 25 for IBD/TNF/PD3, n = 8 for non-IBD/non-TNF/PD4, n = 8 for non-IBD/TNF/PD4, n = 11 for IBD/non-TNF/PD4, n = 6 for IBD/TNF/PD4. PD, postdose.