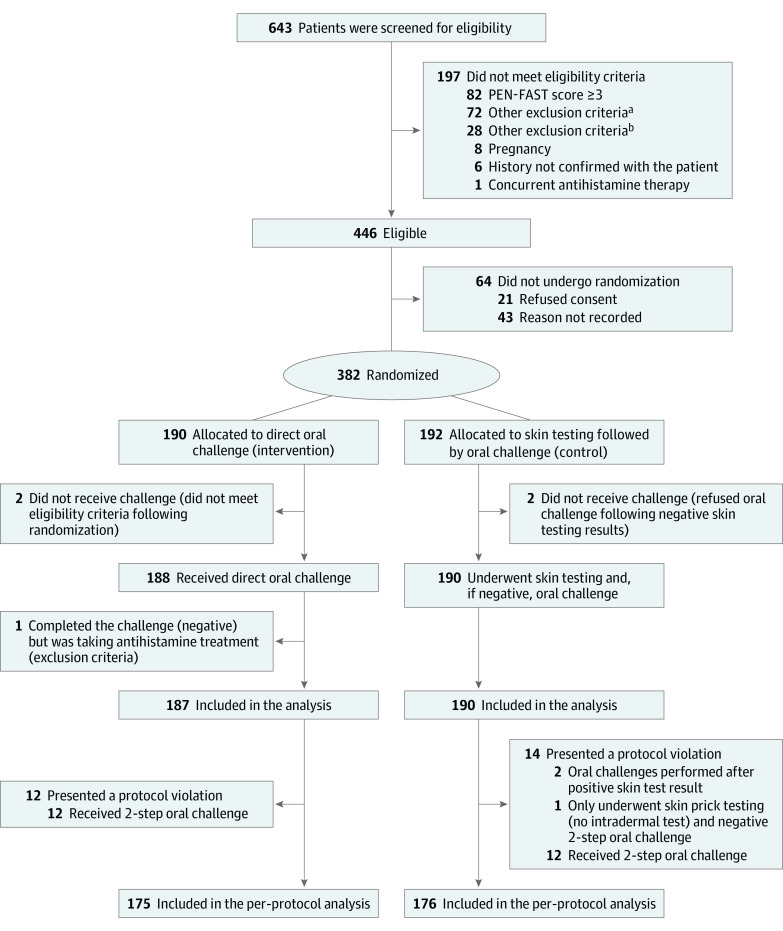
Figure. CONSORT Diagram.
aAny other illness that, in the investigator’s judgment, would substantially increase the risk associated with the patient’s participation in this study, including neurological or psychological conditions.
bPatients with a history of type A adverse drug reaction, drug-associated anaphylaxis, idiopathic urticaria/anaphylaxis, mastocytosis, serum sickness, blistering skin eruption, or acute interstitial nephritis.