Figure 1. Participant Flow in a Trial of Donanemab for Early Symptomatic Alzheimer Disease.
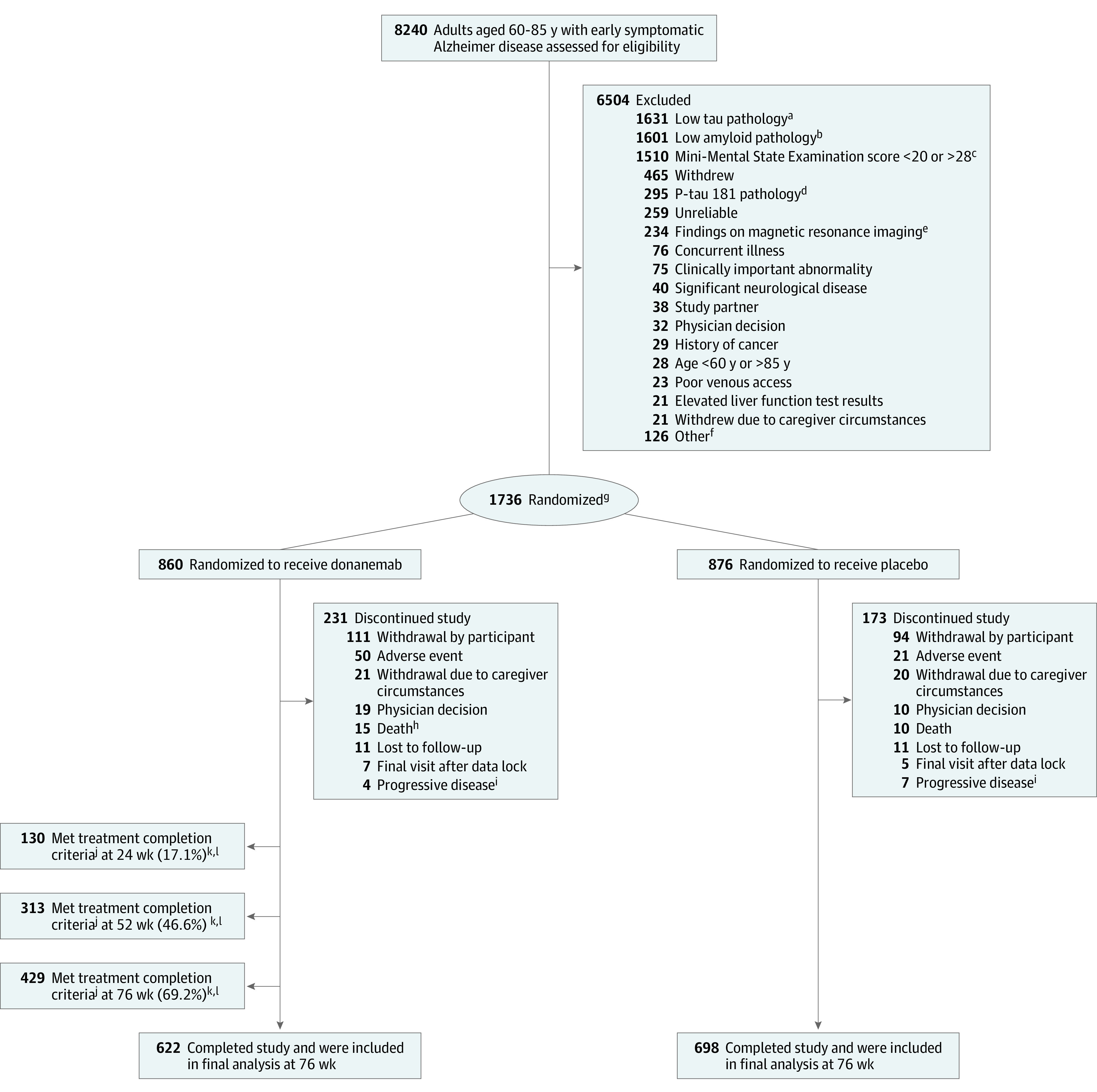
aInclusion criteria for tau pathology: low/medium or high tau indicated by standardized uptake value ratio >1.10 or positive visual read assessed by 18F-flortaucipir positron emission tomography (PET) imaging.
bInclusion criteria for amyloid pathology (≥37 Centiloids) assessed with 18F-florbetapir or 18F-florbetaben PET.
cInclusion criteria for Mini-Mental State Examination: score of 20 to 28.
dPhosphorylated tau 181 (P-tau181) screening criterion was not implemented for the entire trial duration (eMethods in Supplement 3).
eExclusion criteria for MRI include presence of amyloid-related imaging abnormalities of edema/effusion, >4 cerebral microhemorrhages, >1 area of superficial siderosis, and any intracerebral hemorrhage >1 cm or severe white matter disease.
fSummary of other screen failure can be found in eTable 3 in Supplement 3 (lists reason if ≥20 participants).
gStratified by baseline tau categorization and enrolling sites.
hOne additional death occurred after treatment completion and in the follow-up period.
iAlzheimer disease progression to a degree prompting study discontinuation, per investigator judgment.
jTreatment completion criteria: amyloid plaque level of 11 Centiloids on any single scan or 11 to <25 Centiloids on 2 consecutive scans.
kParticipants who met treatment completion criteria are included in discontinuation and completion numbers.
lPercentage calculated as No./total No. of participants with a PET scan at visit: n = 761 at 24 wk, n = 672 at 52 wk, and n = 620 at 76 wk. Corresponding number of participants and percentages for the low/medium tau population were 20.3% (n = 106) at 24 wk, 51.9% (n = 241) at 52 wk, and 73.5% (n = 321) at 76 wk.
