Figure 2. Integrated Alzheimer Disease Rating Scale (iADRS) and Sum of Boxes of the Clinical Dementia Rating Scale (CDR-SB) From Baseline to 76 Weeks.
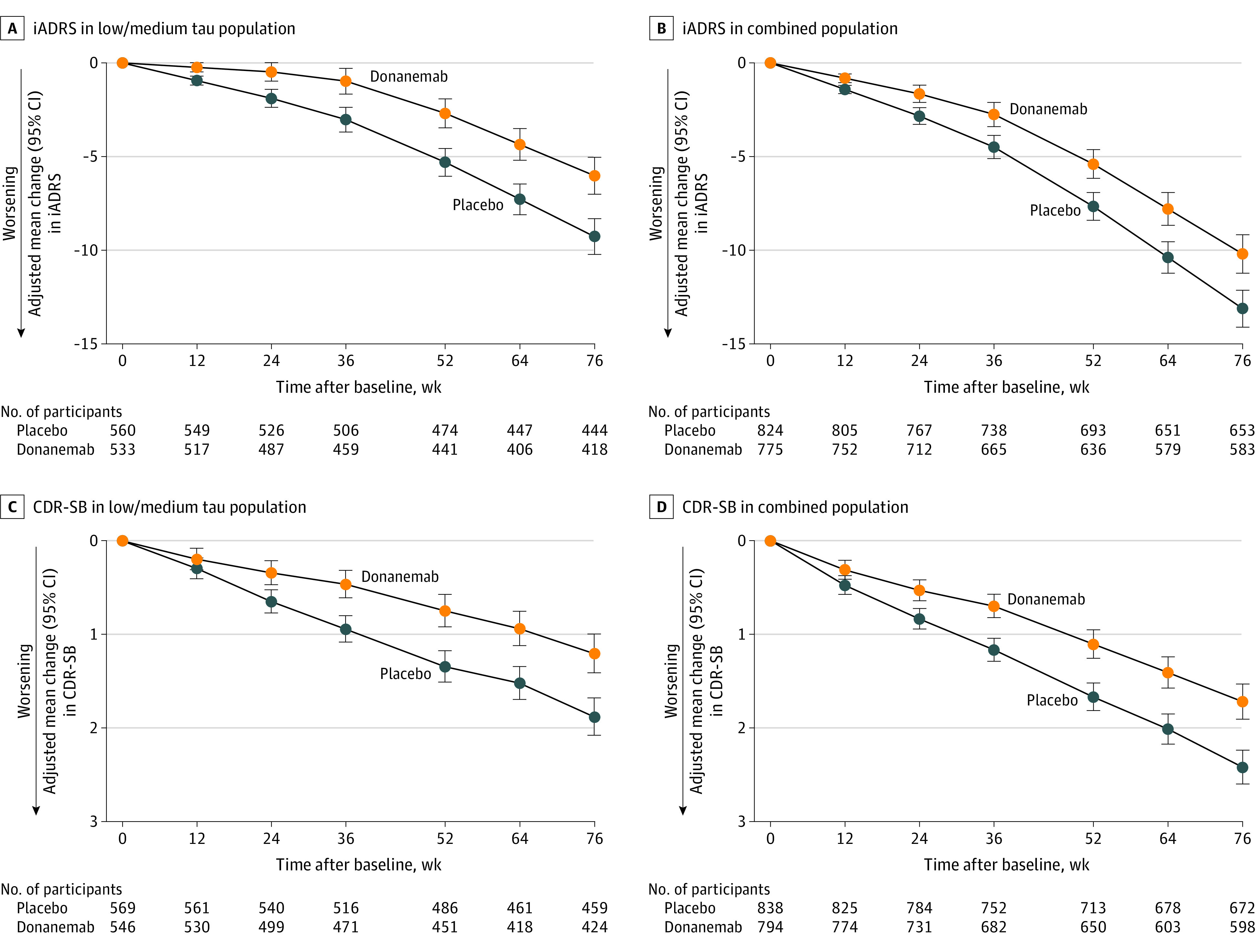
A, 35.1% slowing (95% CI, 19.90%-50.23%) of clinical progression. B, 22.3% slowing (95% CI, 11.38%-33.15%) of clinical progression. C, 36.0% slowing (95% CI, 20.76%-51.15%) of clinical progression. D, 28.9% slowing (95% CI, 18.41%-39.44%) of clinical progression. iADRS data were analyzed using the natural cubic spline model with 2 degrees of freedom (NCS2) and CDR-SB data were analyzed with mixed models for repeated measures (MMRM). For MMRM analyses, 95% CIs for least-squares mean changes were calculated with the normal approximation method. For the Alzheimer Disease Cooperative Study—Instrumental Activities of Daily Living, 13-item cognitive subscale of the Alzheimer Disease Assessment Scale, and CDR-SB clinical assessments analyzed with NCS2, see eFigure 1 (low/medium tau population) and eFigure 2 (combined population) in Supplement 3 and Table 2. For all clinical assessments analyzed with MMRM, see eFigure 3 (low/medium tau population) and 4 (combined population) in Supplement 3 and Table 2. P < .001 for all 76 week time points.
