Figure 3. Extension 3 (aa 325–365) of NADK2 mediates its dimerization and is required for its catalytic activity.
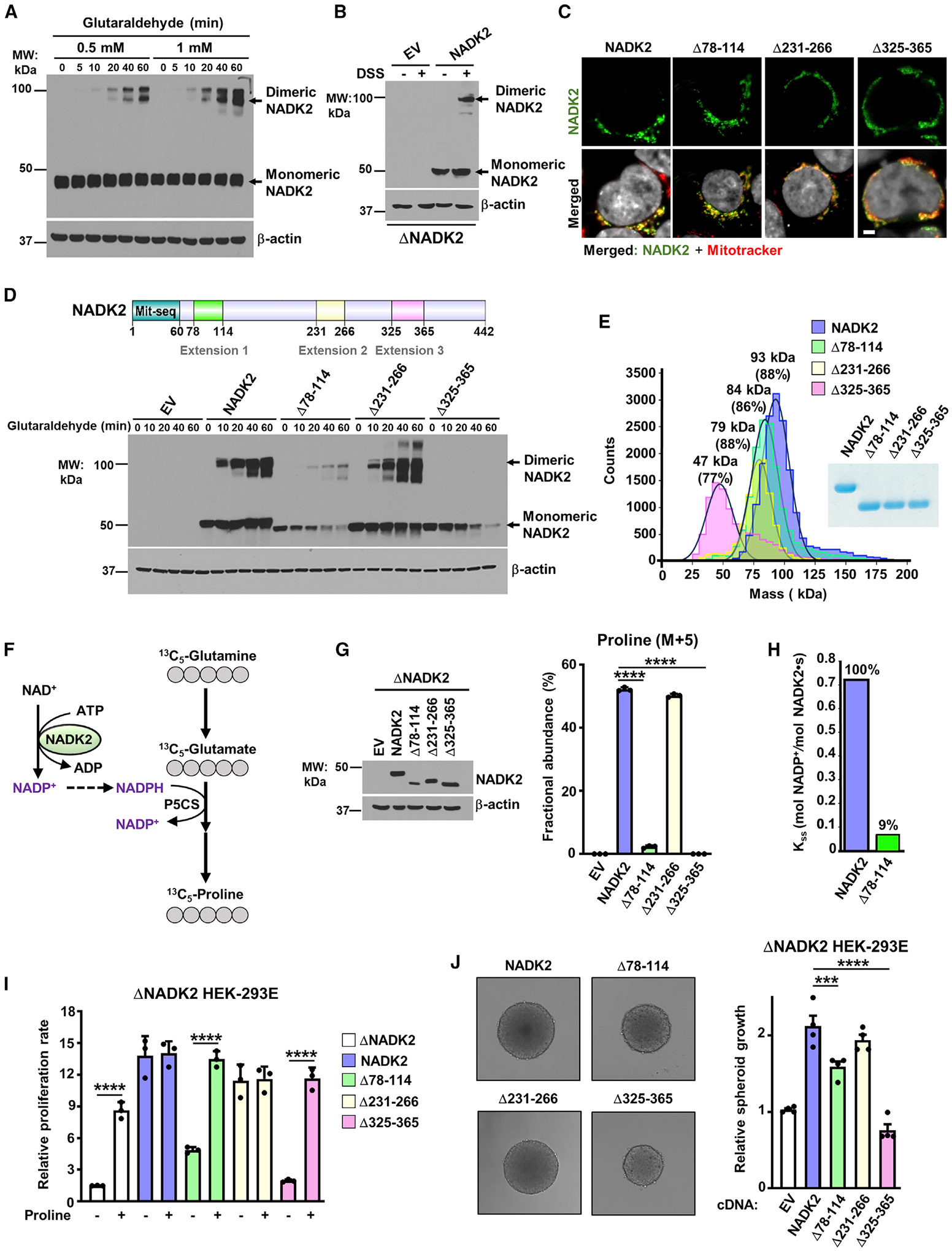
(A) Immunoblots from HEK293E cell extracts incubated with 0.5- or 1-mM glutaraldehyde for the indicated times. Monomeric and dimeric NADK2 and the β-actin control are shown.
(B) Immunoblots as in (A), but from ΔNADK2 HEK293E cells transiently transfected with either empty vector (EV) or NADK2 cDNA and treated with 1-mM DSS for 1 h.
(C) Mitochondrial localization of NADK2 extension-deficient variants. Representative images of localization of the indicated NADK2 variants (green) to mitochondria (red) detected by immunofluorescence with a NADK2 antibody. ΔNADK2 HEK293E cells were transiently transfected either empty vector (control), NADK2 wild type, or NADK2 variants with the indicated deleted extensions (Δ78–114, Δ231–266, and Δ325–365). Mitochondria were stained with MitoTracker (red) and nuclei with Hoechst (gray). Scale bars, 2 μm.
(D) Top: schematic of NADK2 depicting its mitochondrial localization sequence and the unique NADK2 extensions (aa 78–114, aa 231–266, and aa 325–365). Bottom: immunoblots as in (A), but from ΔNADK2 HEK293E cells that were transiently transfected with either empty vector (EV), NADK2 WT, or NADK2 variants with the indicated deleted extensions. Monomeric and dimeric NADK2 and the β-actin control are shown.
(E) Mass photometry of recombinant His-NADK2 (aa 61–442) or the indicated variants purified from E. coli, indicating that His-NADK2Δ325–365 exist in a monomeric state (calculated MW = 47 kDa for 77% of the sample), while NADK2, His-NADK2Δ78–114, and His-NADK2Δ231–266 are dimeric with the indicated molecular weight.
(F) Schematic of 13C5-Glutamine (M+5) tracing to assess the newly synthesized 13C5-proline. 13C-labeled carbons are shown in gray. The rate-limiting pyrroline-5-carboxylate synthase (P5CS) that requires NADK2 activity and mitochondrial NADPH is shown.
(G) Immunoblots and fractional abundance of proline (M+5) from ΔNADK2 HEK293E cells stably expressing either empty vector, NADK2 WT, or NADK2 variants with the indicated deleted extensions (Δ78–114, Δ231–266, Δ325–365) labeled for 3 h with 13C5 glutamine.
(H) Enzymatic activity of human recombinant His-NADK2 and NADK2Δ78–114 variant in the presence of 1 mM NAD+ and 5 mM MgATP.
(I) Cell proliferation of ΔNADK2 HEK293E cells stably expressing either empty vector, NADK2 WT, or the indicated NADK2 variants. Cells were grown for 72 h in 10% dialyzed serum in the presence or absence of 0.2 mM proline.
(J) Representative images and quantification of spheroid size from ΔNADK2 HEK293E cells stably expressing either NADK2 WT or the indicated NADK2 variants that were cultured in 10% dialyzed serum without proline. Quantification of the spheroid size was performed from 5 independent images.
Data are presented as the mean ± SD from n = 3 (G and I) or n = 4 (J) of biologically independent samples, and data are representative of at least two independent experiments. (G, I, and J) ***p < 0.001, ****p < 0.001 for multiple comparisons calculated using one-way ANOVA and Tukey’s post hoc test.
