Figure 5. Regulation of NADK2 functions by post-translational modifications.
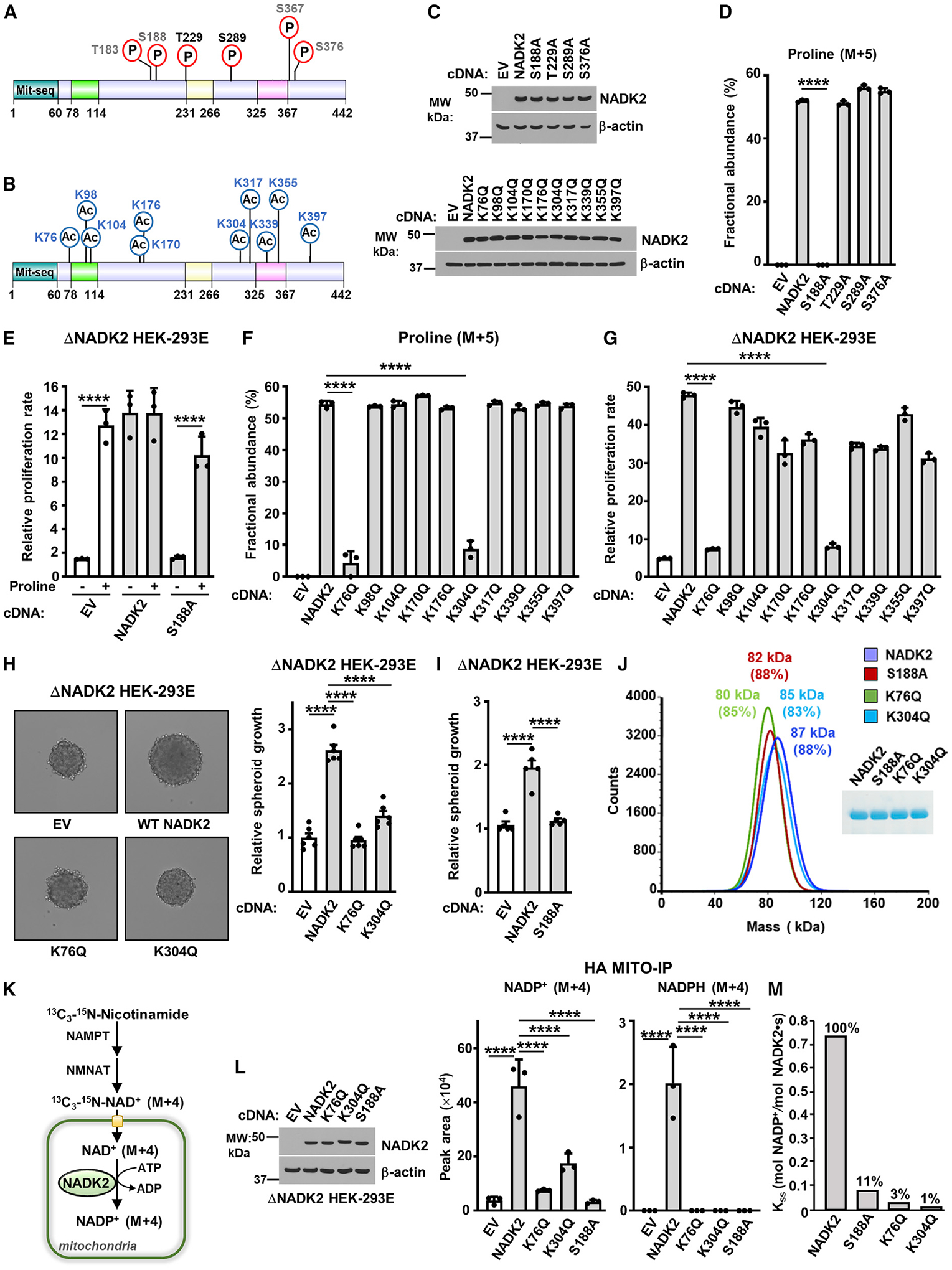
(A) Schematic of NADK2 as in Figure 3D, but showing phosphorylated residues identified in this study (black) or those curated from PhosphoSitePlus (gray). See Table 1.
(B) As in (A), but showing the acetylated residues found on NADK2 from HEK293E cells transiently expressing NADK2-FLAG (this study). See Table 1.
(C) Immunoblots of NADK2 variants from ΔNADK2 HEK293E cells stably expressing the indicated NADK2 phospho-mutants (S or T to A) or acetylation-mutants (K to Q).
(D) Fractional abundance of proline (M+5) as in Figure 3G, but from ΔNADK2 HEK293E cells stably expressing either empty vector, WT NADK2, or the indicated NADK2 variants (S or T to A) labeled for 3 h with 13C5-glutamine.
(E) Relative cell proliferation of ΔNADK2 HEK293E cells stably expressing either empty vector (EV), WT NADK2, or NADK2 S188A. Cells were grown for 72 h in 10% dialyzed serum in the presence or absence of 0.2 mM proline.
(F) Fractional abundance of proline (M+5) as in Figure 3G, but from ΔNADK2 HEK293E cells stably expressing either empty vector (EV), WT NADK2, or the indicated NADK2 acetylation-deficient mutants (K to Q) labeled for 3 h with 13C5-glutamine.
(G) Relative cell proliferation of ΔNADK2 HEK293E cells stably expressing either empty vector (EV), WT NADK2, or the indicated NADK2 acetylation-deficient mutants (K to Q). Cells were grown for 72 h in 10% dialyzed serum in the absence of 0.2 mM proline.
(H) Representative images and quantification of spheroid size from ΔNADK2 HEK293E cells stably expressing either empty vector, NADK2 WT, NADK2 K76Q, or K304Q mutants cultured without proline. Quantification of the spheroid size from 6 independent images for each condition is shown.
(I) Quantification of spheroid size from ΔNADK2 HEK293E cells stably expressing either WT NADK2 or NADK2 S188A cultured without proline. Quantification of the spheroid size from 5 independent images for each condition is shown.
(J) Mass photometry of recombinant His-NADK2 or the indicated variants purified from E. coli, showing that NADK2, His-NADK2 S188A, His-NADK2 K76Q, or His-NADK2 K304Q are dimers. Coomassie blue of purified NADK2 variants is shown on the right.
(K) Schematic of labeling with 13C3-15N-Nicotinamide (M+4), depicting the synthesis of mitochondrial NADP+ (M+4) from NADK2. NAD+ salvage enzymes nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyl transferase (NMANT) are shown.
(L) Immunoblots and normalized peak area of mitochondrial, NADP+ (M+4), and NADPH (M+4) from labeling with 13C3-15N-Nicotinamide (M+4), as illustrated in (K). ΔNADK2 HEK293E cells stably expressing HA-Mito were transiently transfected with EV, WT NADK2, or NADK2 K76Q, K304Q, or S188A mutants. HA-tagged mitochondria were immunoprecipitated (HA-Mito IP) from equal amount of protein for each condition.
(M) Enzymatic activity of human recombinant His-NADK2 and NADK2Δ78–114 variant in the presence of 1 mM NAD+ and 5 mM MgATP.
Data are presented as the mean ± SD from n = 3 (D–G and L) or n = 5 (H), n = 6 (I) of biologically independent samples. Data are representative of at least two independent experiments. (D–G, H, I, and L) ****p < 0.001 for multiple comparisons were calculated using one-way ANOVA and Tukey’s post hoc test.
