FIG 1.
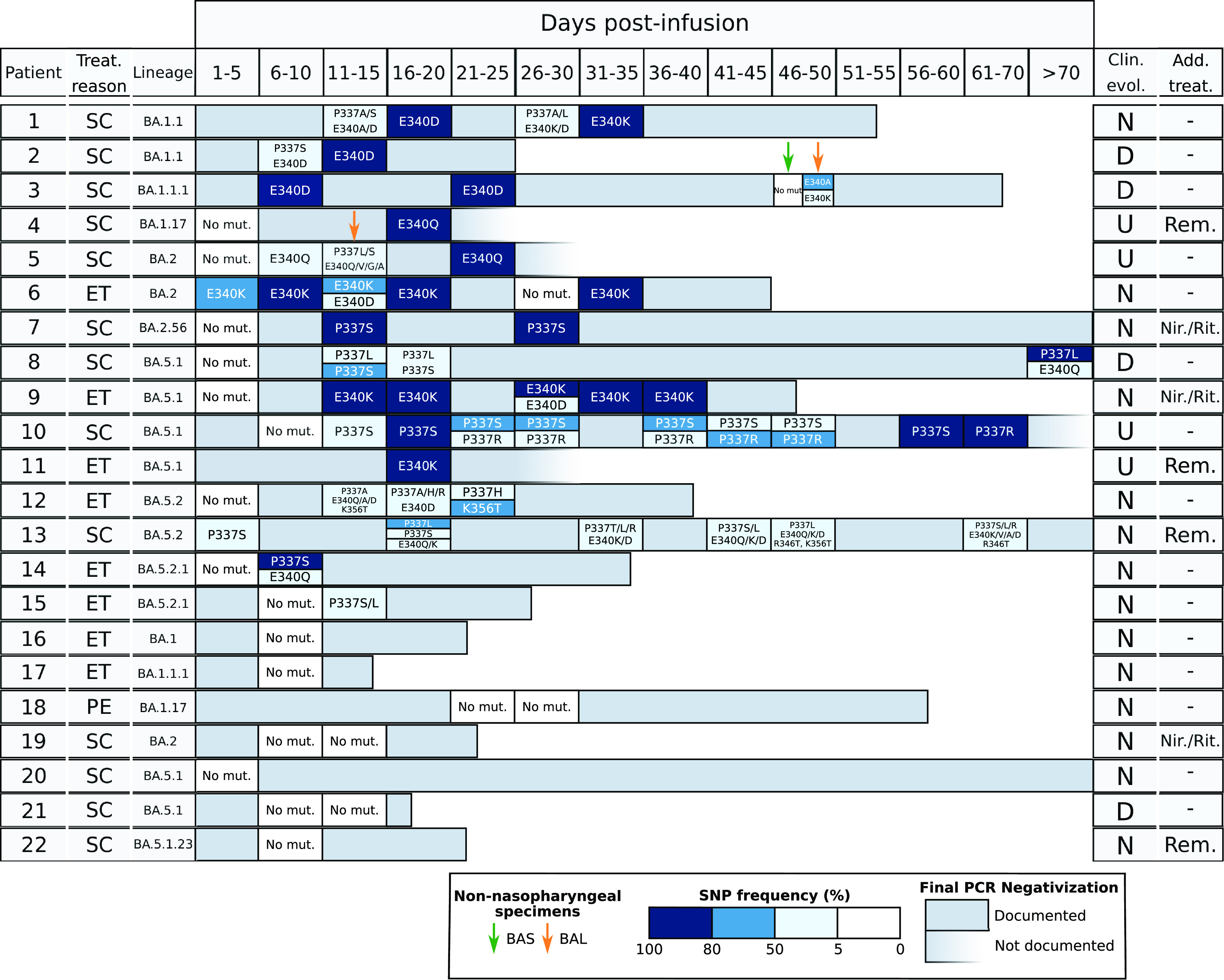
Sotrovimab resistance mutations identified in the patients in study. Each horizontal bar corresponds to the reverse transcription-PCR (RT-PCR) positivity period for each patient. For the specimens which were sequenced, boxed cells indicate whether or not mutations were detected; color gradient indicates the allele frequencies at which the single-nucleotide polymorphisms (SNPs) were identified. The reasons for Sotrovimab treatment (Treat. reason) are indicated as follows: SC, severe COVID; ET, early treatment; PE, SARS-CoV-2 persistence. Clinical evolution (Clin. evol.) is shown as follows: D, died; N, negativization documented by RT-PCR; and U, undefined negativization due to lack of a negative PCR. Additional antiviral treatments (Add. Treat) other than Sotrovimab are shown as follows: Rem, remdesivir; Nir./Rit., nirmatrelvir/ritonavir (Paxlovid). Bronchoalveolar lavage fluid (BAL fluid)/bronchial aspirate (BAS) specimens are indicated by yellow and green arrows, respectively.
