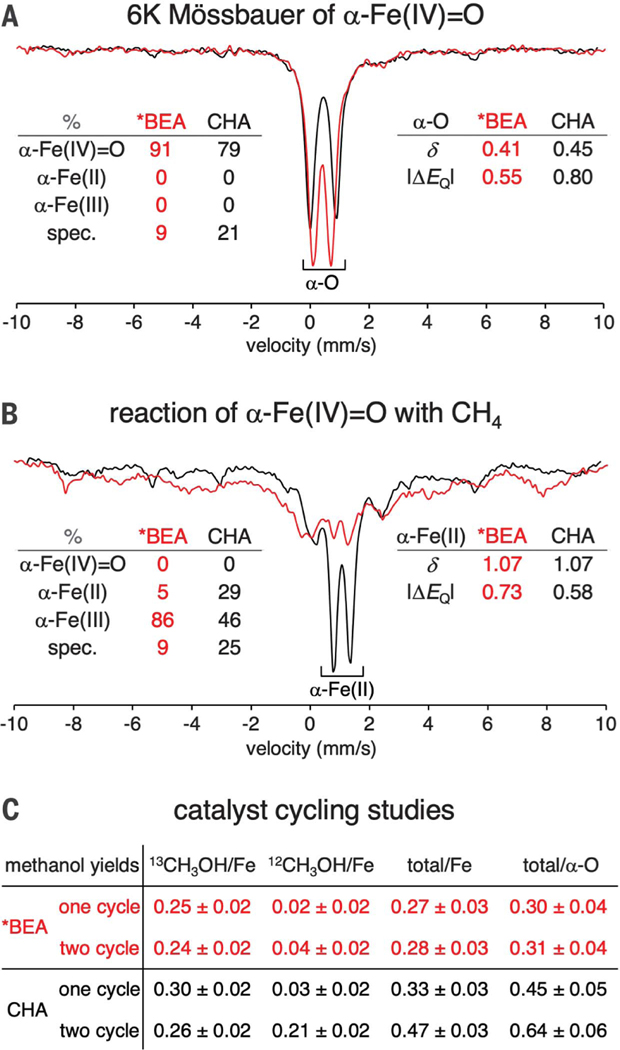
Fig. 2. Effect of lattice topology on active-site regeneration.
(A) Normalized Mössbauer spectra of N2O-activated Fe-*BEA (red) and Fe-CHA (black) at 6 K. Spectral contributions from each Fe oxidation state are quantified at the left (spec. = spectator components that do not contribute to reactivity). Parameters of the α-Fe(IV)=O components are indicated at the right. (B) Normalized Mössbauer spectra of N2O-activated Fe-*BEA (red) and Fe-CHA (black) reacted with CH4 at 300 K and then cooled to 6 K for data collection. Spectral contributions from each oxidation state of the active site are quantified at the left. Parameters of the α-Fe (II) components are indicated at the right. See fig. S1 for details of quantification. The given quantifications have an error of ±5%. δ = isomer shift, ΔEQ = quadrupole splitting (values given in mm/s). (C) Comparison of methanol yields extracted after one reaction cycle with 13CH4 versus two cycles (13CH4, then 12CH4). Yields based on initial α-Fe(IV)=O content make use of Mössbauer quantifications shown in fig. S2.