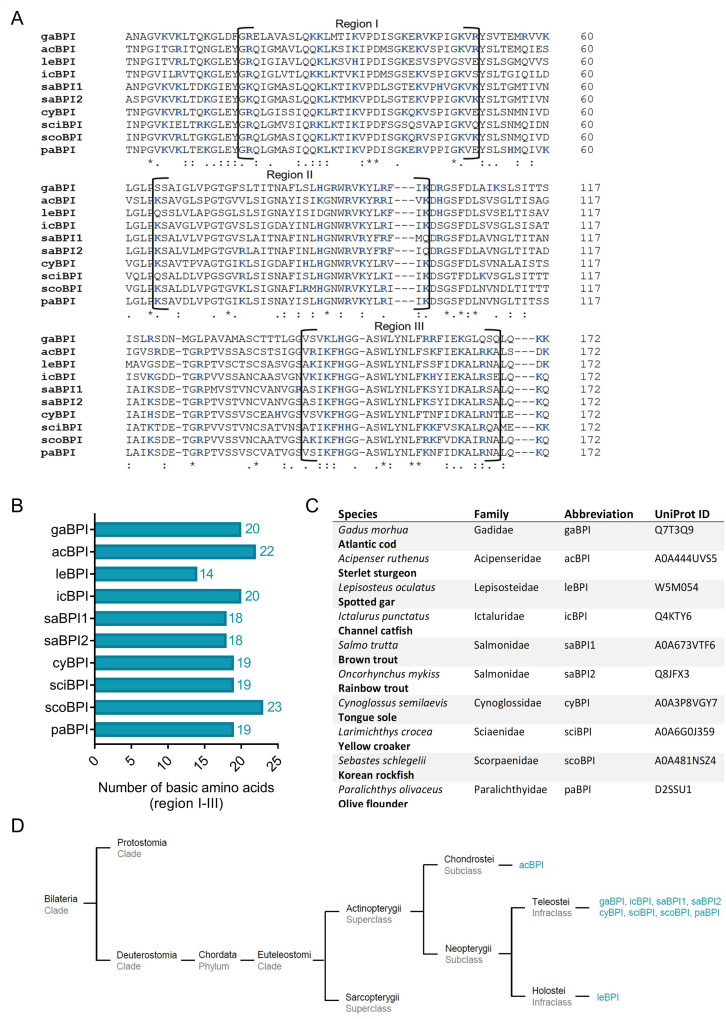
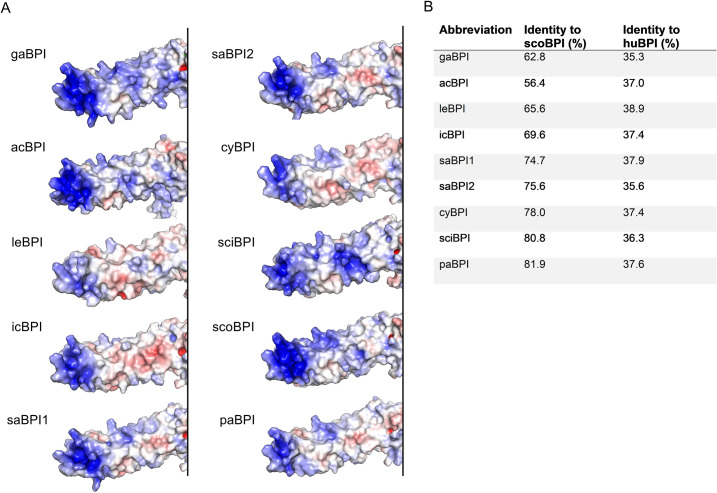
Figure 1. Sequence alignment and analysis of orthologous proteins to human bactericidal/permeability-increasing protein (huBPI).
(A) Sequence alignment of amino acid sequences of orthologous proteins. Functional regions I–III are framed, and positively charged amino acids are shown in blue. The amino acid sequence mediating the bactericidal activity of human BPI (huBPI) is underlined. (B) Functional regions I–III (dark grey) as shown for huBPI. (C) Phylogenetic tree analysis mapping BPI orthologous proteins to their respective taxonomic ranks. (D) Number of basic amino acids in regions I–III as shown for oyster BPI (osBPI), scorpionfish BPI (scoBPI), huBPI and murine BPI (muBPI). (E) Electrostatic surface potentials calculated for the N-terminal barrel of osBPI, scoBPI, huBPI, and muBPI. Negatively charged areas are colored in red, and positively charged domains are shown in blue.