Abstract
Ample evidence suggests that acute stress can worsen symptom severity in Tourette syndrome (TS); however, the neurobiological underpinnings of this phenomenon remain poorly understood. We previously showed that acute stress exacerbates tic-like and other TS-associated responses via the neurosteroid allopregnanolone (AP) in an animal model of repetitive behavioral pathology. To verify the relevance of this mechanism to tic pathophysiology, here we tested the effects of AP in a mouse model recapitulating the partial depletion of dorsolateral cholinergic interneurons (CINs) seen in post-mortem studies of TS. Mice underwent targeted depletion of striatal CINs during adolescence and were tested in young adulthood. Compared with controls, partially CIN-depleted male mice exhibited several TS-relevant abnormalities, including deficient prepulse inhibition (PPI) and increased grooming stereotypies after a 30-min session of spatial confinement - a mild acute stressor that increases AP levels in the prefrontal cortex (PFC). These effects were not seen in females. Systemic and intra-PFC AP administration dose-dependently worsened grooming stereotypies and PPI deficits in partially CIN-depleted males. Conversely, both AP synthesis inhibition and pharmacological antagonism reduced the effects of stress. These results further suggest that AP in the PFC mediates the adverse effects of stress on the severity of tics and other TS-related manifestations. Future studies will be necessary to confirm these mechanisms in patients and define the circuitry responsible for the effects of AP on tics.
Subject terms: Psychiatric disorders, Sensorimotor processing
Introduction
Tics are sudden, non-rhythmic, stereotypical movements or utterances characterized by variable intensity and complexity. The most disabling tic disorder, Tourette syndrome (TS), is a childhood-onset condition characterized by multiple motor tics and at least one phonic tic for over one year [1]. TS is complicated by the high prevalence of comorbid psychiatric disorders, such as obsessive-compulsive disorder (OCD), attention-deficit hyperactivity disorder (ADHD), anxiety, and depression [2, 3]. Current pharmacotherapies for TS remain unsatisfactory. The main pharmacological treatments for TS, dopaminergic antagonists and α2 adrenergic agonists [4], are associated with inconsistent efficacy and significant adverse effects [5–7].
Tics fluctuate in number, frequency, intensity, and complexity [8]. While the biological causes of these fluctuations remain elusive, several lines of evidence point to environmental stress as a crucial influence on tic severity. For example, tic severity is associated with stressful life events [9, 10]. This relationship has been confirmed by longitudinal analyses, which have documented that cumulative psychosocial stress predicts future tic severity [11]. Furthermore, tic severity is correlated with self-report ratings of daily stress [12] and recent adverse events [13]. Tics can be exacerbated by specific stressors, such as hypostimulation and fatigue [14]; the contribution of acute psychosocial stress may be more complex [15].
Tic disorders are associated with anatomical and functional alterations within the cortico- striatal-thalamo-cortical circuitry. The causes of these alterations remain poorly understood. Several studies have documented a selective loss in cholinergic and parvalbumin-positive GABAergic interneurons in the dorsal striatum of individuals with severe TS [16–18]. It is possible that a local reduction in striatal interneurons (perhaps due to genetic and early-life inflammatory factors) may lead to the formation of “focal disinhibition areas” in the striatum [19]. Abnormal activation of these foci may lead to the involuntary execution of tics.
Building on these observations, Xu et al. [20] modeled the loss of striatal cholinergic interneurons (CINs) using a recombinant virus (A06) expressing the simian diphtheria toxin (DT) receptor in Cre-expressing cells. Stereotactic injection of A06 in the dorsal striatum of choline acetyltransferase (ChAT)-cre mice was followed by systemic DT administration, resulting in a selective ablation of ~50% striatal CINs, which spared all other local cell types [20]. This manipulation produced baseline impairments in rotarod performance but no overt alterations in TS-relevant responses; however, it predisposed male mice to exhibit grooming stereotypies in response to acute stress [20]. The lack of baseline behavioral deficits in these mice may partially reflect the fact that CINs were depleted in adulthood following normal development; this may have missed critical neurodevelopmental components. On the other hand, the stress sensitivity seen in these animals is strikingly reminiscent of the worsening of tics and other symptoms after stress in TS patients. The mechanisms underlying these relationships remain unclear.
Growing evidence suggests that the pathophysiology of TS may be modulated by neurosteroids [21]. These mediators play a crucial role in regulating the stress response [22–24]. Using a different mouse model of TS with high face and predictive validity, the D1CT-7 mouse, we have shown that stress exacerbates tic-like behaviors as well as other TS-relevant effects [25] through the action of the neurosteroid allopregnanolone (AP; 3α, 5α-tetrahydroprogesterone) [26]. This metabolite of progesterone is increased in response to acute stress [22] and activates GABAA receptors [27, 28]. AP elicited tic-like movements, increased stereotyped behavior, and reduced prepulse inhibition (PPI) in D1CT-7 mice [26]. Furthermore, isoallopregnanolone (isoAP), the 3β-epimer of AP that acts as an antagonist to the AP-binding site within GABAA receptors [29, 30], countered the adverse effects of stress on tic-like behaviors and PPI deficits in this model [31].
The present study was designed to model the impact of early-life CIN depletion in mice and assess its implications for sensorimotor gating and stress susceptibility. To test the effects of AP in this model, we tested whether this neurosteroid can aggravate tic-like stereotypies and whether antagonizing its actions can block the effects of acute stressors. This extends our previous work in the D1CT-7 mouse, which has limited construct validity, to a model of TS pathophysiology that recapitulates a neuropathological abnormality associated with refractory tics [16, 17].
Materials and methods
Animals
Homozygous ChAT-cre female mice (B6; 129S6- ChATtm2(cre)Lowl/J obtained from The Jackson Laboratory, 006410) were crossed with C57BL/6J male mice (The Jackson Laboratory, 000664; Bar Harbor, ME). Male and female hemizygous ChAT-cre transgenic mice were produced in our animal facilities by crossing hemizygous male ChATtm2(cre)Lowl/J mice with female wildtype C57BL/6J mice or hemizygous female ChATtm2(cre)Lowl/J mice with male wildtype C57BL/6J mice. Genotypes were confirmed by PCR. Experiments were performed using adult male and female mice (2–4 months old). All animals were allowed to acclimate to facilities for 7–10 days before behavioral testing. Housing facilities were maintained at 22 °C. Most mice were kept in husbandry facilities with a reversed 12-hour light/dark cycle (with lights switching at 6:00 p.m. and at 6:00 a.m.). However, to control for potential circadian effects in the characterization of spontaneous and stress-related responses, separate groups of animals were housed in separate facilities with a regular light/dark cycle. Under both conditions, experimental manipulations were carried out between 9:00 a.m. and 5:00 p.m. Every effort was made to minimize the number and suffering of animals. Thus, animal numbers for each experiment were determined through power analyses conducted on preliminary results. All experimental procedures were approved by the local Institutional Animal Care and Use Committees.
Drugs
AP (Tocris Bio-Techne, Minneapolis, MN) and finasteride (AstaTech, Bristol, PA) were dissolved in 2.5% DMSO, 2.5% Tween 80, and 0.9% NaCl. IsoAP (3β,5α, tetrahydroprogesterone) was provided by Asarina Pharma AB (Solna, Sweden), suspended in 3% hydroxypropyl β-cyclodextrin, and administered IP. For regional intracerebral infusions, AP was dissolved in a Tween 80/Ringer solution (final concentration, 1:1, v:v); AP was then infused in a cyclodextrin/Ringer solution (final concentration 1:5, v-v). For regional intracerebral studies, isoAP was infused in Ringer solution (final concentration 1:1, v:v). The D1 receptor antagonists ecopipam (ECO) and SCH23390, the D2 receptor antagonist haloperidol (HAL), and the α2-adrenergic agonist clonidine (CLO) (Sigma Aldrich, St. Louis, MO) were dissolved in 0.9% NaCl and administered IP. The injection volume for all systemic administrations was 10 ml/kg.
CIN depletion procedure
CIN depletion was induced in ChAT-cre hemizygous mice using a modified version of the protocol described by Xu et al. [20], specifically designed to enable selective CIN depletion in early developmental stages. Male and female pups at postnatal day (PND) 4 underwent bilateral stereotaxic infusion of either A06 (a viral construct harboring the simian DT receptor) or its control (C06) [20]. Briefly, after anesthesia was induced in a pup by hypothermia, its head was gently placed between the ear bars of a neonatal stereotaxic frame (Stoelting; Wood Dale, IL). The following coordinates were used to target the striatum: AP − 0.5 mm; ML ± 1.6 mm; DV − 2.8 mm. A 5 µl Hamilton Neuros syringe (Reno, NV) was used to inject 0.25 μl of viral construct or control vector bilaterally. The syringe was left in place for at least 2 min after completion of the infusion to reduce backflow. The pup was then placed on a warming pad until its body temperature and skin color returned to normal, and afterward, it was returned to its mother. Following surgery, mice were left undisturbed in their home cage until PND 18, when they received a single intraperitoneal (IP) injection of DT (Sigma, St. Louis, MO) at a dose of 1 μg/kg. Mice were weaned at PND 21 and group-housed in same-sex sibling pairs until experimental procedures.
Behavioral procedures
A total of 466 male and female mice were used in the present study. Partially CIN-depleted (CIN-d; treated with A06) and control (treated with C06) mice were first tested using a comprehensive battery of behavioral tasks aimed at capturing responses related to TS and comorbid disorders. This battery included the assessment of (1) spontaneous and stress-induced grooming behavior, (2) eye blinks and head movements; (3) locomotor activity in an open field; (4) startle reflex and prepulse inhibition (PPI); (5) marble-burying responses; and (6) elevated-plus maze behavior. These tests were performed as previously detailed [25, 31–35] (for a detailed description, see Supplementary Materials) both in baseline conditions and in response to acute stress. Then, the effects of different classes of effective treatments for tic disorders were measured to verify whether TS-related abnormalities were responsive to TS therapies. Following verification that spatial confinement increased AP levels in the medial prefrontal cortex (mPFC), the behavioral effects induced by systemic and local injections of this neurosteroid were assessed. Finally, CIN-d and control mice were subjected to pharmacological manipulations that either reduced AP synthesis or antagonized its effects, to verify whether these drugs may significantly reduce the behavioral effects of stress in CIN-d mice. Before all behavioral testing, mice were consistently habituated to the experimental room and an empty adjacent room for at least 60 min per day. On the day of the test, mice were carried to the waiting room for at least 60 min, and then one animal at a time was brought to the experimental room using a clean transport cage. Both the waiting and the testing rooms were kept at 100 lux (dim light); however, light intensity was occasionally raised to 1000 lux to study the impact of stressful, anxiogenic conditions on marble burying and elevated plus maze (see below). Each test was conducted with 8 mice/group, except for the analyses of the effects of isoAP and marble-burying in the light phase, which were performed with 6 animals/group. For each test, mice of similar weight and age were selected randomly. Each mouse did not undergo more than three behavioral tests, conducted at least one week apart from each other. A synoptic view of all experimental procedures is shown in Supplementary Fig. 1.
Stereotaxic surgery for regional intracerebral studies
CIN-d and control mice were deeply anesthetized with xylazine/ketamine (20·80 mg/kg, IP) and then placed into a stereotaxic frame (David Kopf Instruments, Tujunga, CA). The target locations for cannulation were, from bregma: ((i) mPFC: AP + 1.8 mm, ML ± 0.3 mm, DV – 2.5 mm from the skull surface; (ii) dorsolateral striatum (DLS): AP + 0.5 mm, ML ± 2.2 mm, DV – 2.2 mm, according to the coordinates of Paxinos and Watson [36]. Mice were allowed to recover in their home cages (single-housed), and after one week, they received bilateral microinjections in the targeted area (according to the specific experimental design). Microinjections were performed by gently restraining the mouse, removing the stylet, and replacing it with the injector (P1 Technologies Inc.) connected by a 250 μl Hamilton syringe via PE tethers. A microinfusion pump (KD Scientific, Holliston, MA) delivered 0.5 µl/side of drug solution (or its vehicle solution) at a constant flow/rate of 0.25 µl/min. After infusion, the injector was left in place for 2 min to allow fluid diffusion. After behavioral tests, animals were sacrificed, and the histological verification of the cannula location was confirmed. Animals with errant locations of either device or damage to the targeted area(s) were excluded from the analysis.
Neurosteroid analyses
Extraction, derivatization, and GC-MS analyses of neurosteroids were performed as described [37, 38], with minor modifications. Steroids measured included pregnenolone, progesterone, 5α-dihydroprogesterone, and AP. The PFCs of male CIN-d and control mice were harvested, snap-frozen, and homogenized in distilled water. Supernatants were extracted with ethyl acetate, and after lyophilization, neurosteroids were purified and separated by HPLC [37]. Tritiated neurosteroids (American Radiolabeled Chemicals, St. Louis, MO) were added to monitor retention time through HPLC [38] while deuterated internal standards (CDN Isotopes, Pointe-Claire, QC, and Steraloids, Newport, RI) were added to allow quantification of the compound of interest. The HPLC fractions containing pregnenolone, progesterone, and AP were derivatized with heptafluorobutyric acid anhydride (HFBA) (Supelco, Bellefonte, PA). 5α-dihydroprogesterone was derivatized with N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA)-ammonium iodide (NH4I)/1,4-dithioerythritol (DTE)/acetonitrile (CH3CN) (Sigma-Aldrich) in a ratio of 1000/2/5/1000 and subjected to GC-MS. Mass fragmentography analysis of derivatized hormones was performed in the standard electron impact mode with a detection limit of ≈10 fmol and intra-assay coefficients of variation less than 5%. Neurosteroids were identified based on their GC/MS retention time characteristics; the definitive structural identification of each neurosteroid was provided by its unique mass fragmentation pattern. To calculate the quantity of the neurosteroid of interest in each fraction, the area under the peak of the neurosteroid in the sample was divided by the area under the peak of the deuterated internal standard. Only peaks with a signal-to-noise ratio greater or equal to 5:1 were integrated.
Immunohistochemistry and quantification
Mice were sacrificed and perfused with 4% paraformaldehyde in phosphate-buffered saline (PBS); brains were removed and post-fixed overnight, followed by cryoprotection in 30% sucrose in PBS. The brains were then frozen in NEG-50 (Richard-Allan Scientific) tissue freezing medium and sectioned on a Leica cryostat at 30 µm thick and mounted on glass slides. Slides with brain sections containing the striatum (including dorsal and ventral striatum and adjacent septal nuclei; see Supplementary Fig. 2) were incubated in primary antibodies overnight (22–26 hours) – mouse anti-ChAT (Thermo Fischer, MA5-31383) and chicken anti-GFP (Aves, GFP-1020). Sections were then incubated for 2 h in secondary antibodies – goat anti-mouse 568 (Thermo Fischer, A-11031) and goat anti-chicken 488 (Thermo Fisher, A-11039), followed by coverslipping with ProLong Gold with DAPI for nuclear labeling (Thermo Fischer, 36931). Sequential images of the striatum were taken on a Zeiss Scope.A1 using a Plan- APOCHROMAT 10x objective and an Axiocam 503 mono. These images were then stitched together using the Stitching Plugin in FIJI (FIJI is Just ImageJ, Schindelin et al. [39]) using the default values on the Sequential Images setting. Cell counting was then performed with counters blind to sex and treatment of animals in these reconstructed sections.
Statistical analyses
Normality and homoscedasticity of data distribution were verified using Kolmogorov–Smirnov and Bartlett’s tests. Statistical analyses were performed using one- or multiway ANOVA, as appropriate. Post-hoc comparisons were performed by Tukey’s test. Significance thresholds were set at 0.05. While only the main results of statistical analyses are presented in the text, all details are reported in Supplementary Table 1.
Results
Early-life CIN depletion predisposes male, but not female, mice to TS-related behavioral alterations under acute stress
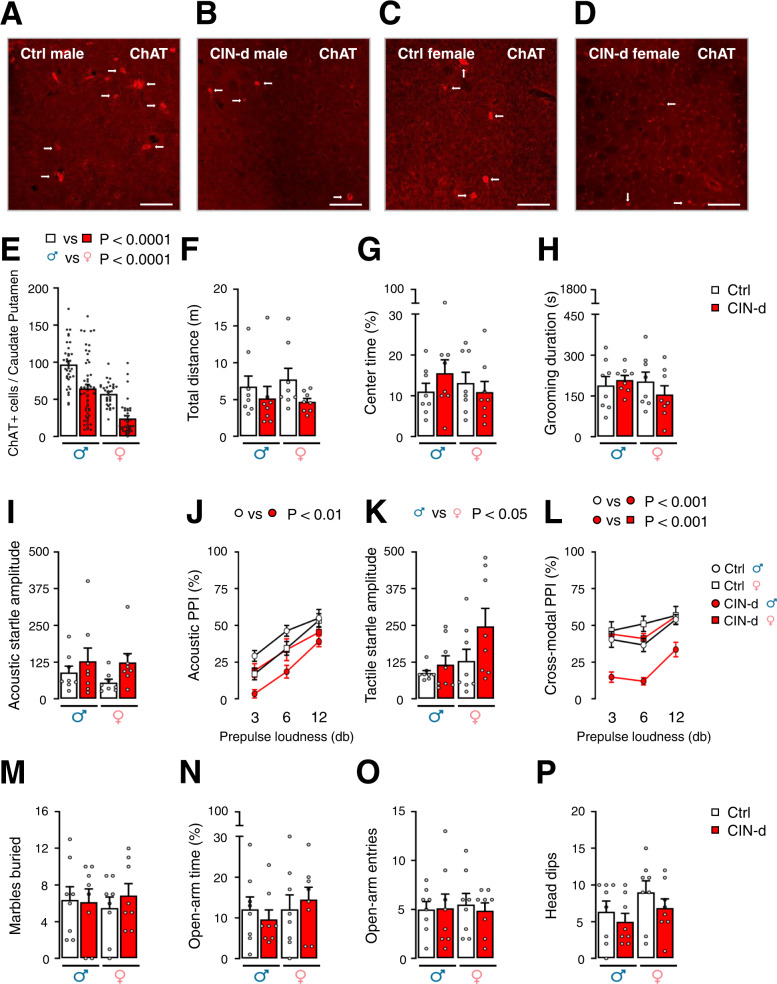
To evaluate the efficacy of CIN depletion, we counted ChAT-positive neurons in the striatum of mice treated with A06, compared to counterparts treated with the control virus C06 (Supplementary Fig. 2A). Because our past work showed sexual dimorphism in the effects of interneuron depletion [40], we examined the impact of this manipulation in male and female mice (Fig. 1 and Supplementary Table 1A). Notably, we found that DT administration to A06-treated mice successfully reduced CIN number in the caudate-putamen of both males and females (Fig. 1A–E and Supplementary Fig. 2B–D) [Main effect of CIN ablation: F(1,155) = 43.90, P < 0.0001; partial η2 = 0.22]. Notably, females had significantly fewer CINs than males [Main effect of sex: F(1,155) = 65.51, P < 0.0001; partial η2 = 0.30], but the effects of CIN depletion were comparable between sexes (Supplementary Table 1A). To verify whether the same surgical manipulation would lead to CIN depletion in other brain regions, we also counted ChAT-positive neurons in the nucleus accumbens and in the septal nuclei of a random subset of male and female mice; these analyses showed that CINs were marginally, yet not significantly reduced in the ventral striatum (Supplementary Figs. 2E–G and 3A, B) [Main effect of CIN-d: F(1,52) = 2.88, P = 0.10; partial η2 = 0.05] while their number was not modified in the septum (Supplementary Figs. 2H–J and 3C, D). Female mice showed a greater number of ChAT-positive neurons than males in the ventral striatum (Supplementary Fig. 3B) [Main effect of sex: F(1,52) = 4.63, P = 0.04; partial η2 = 0.08], independently of CIN depletion (Supplementary Table 1F).
Fig. 1. Effects of early-life cholinergic interneuron depletion (CIN-d) in male and female mice.
A–E Cre-inducible expression of the simian diphtheria toxin (DT) receptor (A06) in the dorsolateral striatum (DLS) of male (A, B) and female C, D ChAT-cre mice. ChAT immunostaining shows that the combination of A06 infection on postnatal day (PND) 4 and systemic DT administration (PND 18) led to a marked reduction of cholinergic interneurons (CINs) in the striatum in comparison with control (Ctrl) male and female mice treated with C06. Scale bars equal 50 µm. E Quantitative differences in ChAT-positive cells in the DLS of male and female CIN-d and Ctrl mice revealed marked losses of CINs in A06-treated mice across both sexes. Additionally, these analyses revealed that the number of dorsal striatal CINs was significantly lower in females, irrespective of the experimental group. F–P Characterization of spontaneous behaviors of juvenile CIN-d and Ctrl mice during the dark phase. CIN-d and Ctrl mice displayed comparable locomotor activity in an open field (F–G) as well as similar total duration of self-grooming behavior (H). While the amplitude of acoustic startle reflex was higher in all CIN-d mice, irrespective of sex (I), the corresponding prepulse inhibition (PPI) was significantly disrupted only in CIN-d males (J). While female mice showed higher tactile startle amplitudes (K), only CIN-d male mice displayed cross-modal PPI deficits (L). Under normal ambient light, CIN-d and Ctrl mice exhibited similar marble-burying behavior (M). Similarly, testing in the elevated plus maze did not reveal significant differences between groups (N–P). Data were analyzed using two-way ANOVA designs, except for acoustic (J) and cross-modal (L) PPI analyses, which were performed by three-way, repeated-measure ANOVAs. All data are shown as means ± SEM. Each behavioral test was conducted with n = 8/group. For further details, see text and Supplementary Table 1A.
We first assessed the behavioral responses of CIN-d and control male mice (n = 8/group) in the dark phase. These experiments revealed no baseline differences in open-field locomotor activity (Fig. 1F–G) or spontaneous grooming in their home cage (Fig. 1H). To investigate whether early-life CIN depletion alters sensorimotor gating, separate groups of mice (n = 8/group) were tested for PPI of the acoustic (Fig. 1I–J) and tactile startle (Fig. 1K–L). Acoustic startle analyses revealed no significant main effects for CIN ablation and sex and no interaction between these factors. However, acoustic PPI was reduced in CIN-d males across all prepulse loudness levels (Fig. 1J) [CIN-d × sex interaction: F(1,28) = 6.21, P = 0.02; partial η2 = 0.33]. Tactile startle (Fig. 1K) was unaffected by partial CIN depletion, even though females had significantly higher startle responses than males [Main effect of sex: F(1,28) = 4.64, P = 0.04; partial η2 = 0.14]. The analysis of cross-modal PPI (Fig. 1L) confirmed a significant impairment of sensorimotor gating in CIN-d males but not females [CIN-d × sex interaction: F(1,28) = 6.43, P = 0.02; partial η2 = 0.38].
To test whether CIN ablation alters other behavioral measures associated with compulsivity and anxiety, we tested a separate group of CIN-d and control mice (n = 8/group) with the marble-burying task and elevated plus-maze under regular environmental light (Fig. 1M–P). No significant effects were found on any of these paradigms (Supplementary Table 1A).
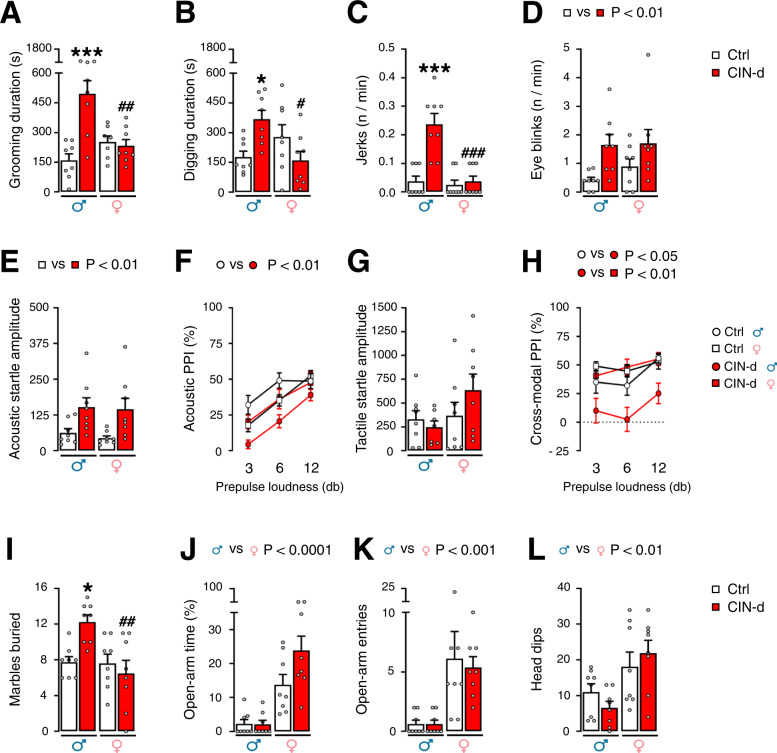
Prompted by these results, we investigated whether exposure to a mild stressor that increases brain AP levels may worsen TS-related behaviors. To this end, we used spatial confinement, a manipulation shown to exacerbate tic-like responses in another model system [25, 26] (Fig. 2 and Supplementary Table 1B). Spatial confinement elicited a significant increase in grooming (Fig. 2A) in CIN-d males compared to male controls and female CIN-d mice (n = 8/group). Conversely, no difference was found between female CIN-d and control mice (Fig. 2A) [CIN-d × sex interaction: F(1,27) = 16.38, P = 0.0004; partial η2 = 0.34]. Similarly, we found that male, but not female CIN-d mice exhibited a significant increase in digging responses (Fig. 2B) [CIN-d × sex interaction: F(1,28) = 10.99, P = 0.003; partial η2 = 0.28] and head jerks (Fig. 2C) [CIN-d × sex interaction: F(1,28) = 15.00, P = 0.0006; partial η2 = 0.35] in response to spatial confinement. Measurements of eye blinks showed that spatial confinement elicited an increase in this response in both CIN-d males and females (Fig. 2D) [Main effect of CIN-d: F(1,28) = 9.61, P = 0.004; partial η2 = 0.25]. Finally, after spatial confinement, separate groups of CIN-d male and female mice (n = 8/group) exhibited a significant increase in acoustic startle amplitude (Fig. 2E) [Main effect of CIN-d: F(1,28) = 12.98, P = 0.001; partial η2 = 0.32]; however, only males displayed a significant PPI deficit in comparison with control males (Fig. 2F) [CIN-d × sex interaction: F(1,28) = 8.02, P = 0.008; partial η2 = 0.25]. While no changes in tactile startle amplitude were found in CIN-d and control mice exposed to spatial confinement (Fig. 2G), a significant interaction between CIN ablation and sex was found in cross-modal PPI [CIN-d × sex interaction: F(1,28) = 4.23, P = 0.04; partial η2 = 0.34] driven by significant differences between CIN-d and control males (P < 0.05) and between CIN-d male and female mice (P < 0.01) (Fig. 2H). While acoustic and tactile PPI were loudness-dependent, the prepulse level did not interact with any other factor (Fig. 2F, H). To assess the impact of high environmental stress on compulsivity, we studied the effects of bright ambient light – a well-established anxiogenic condition for mice – in marble-burying and elevated-plus-maze, using another group of animals (n = 8/group). Notably, these environmental conditions elicited a significant enhancement in marble-burying behaviors in male, but not female, CIN-d mice, as compared to their controls (Fig. 2I) [CIN-d × sex interaction: F(1,28) = 7.61, P = 0.01; partial η2 = 0.21]. However, no significant effects of CIN-d were observed in the elevated plus maze in either sex (Fig. 2J–L). However, it should be noted that, in this bright-light condition, both CIN-d and control males manifested high anxiety-like behavior, as signified by the failure of most males to enter open arms. Conversely, female mice surprisingly displayed less anxiety-like responses, with more open-arm entries and time. While anxiety-like behaviors were significantly milder in females, no differences were found between CIN-d and control mice.
Fig. 2. Effects of acute stress on the behavior of CIN-depleted (CIN-d) and control (Ctrl) male and female mice under the dark phase.
Exposure to spatial confinement (SC; 40-min duration) led to significantly higher total durations of A self-grooming and B digging responses in male, but not female, CIN-d mice. In a separate experiment, subjecting mice to a 10-min SC session led to a greater frequency of C head jerks in CIN-d males and D eye blinks in CIN-d male and female mice. SC (40 min, within a familiar cage) led to E an increased acoustic startle reflex in CIN-d mice of both sexes and F disrupted acoustic prepulse inhibition (PPI) in CIN-d males, as compared with Ctrl males. The same stressor elicited no differences in G the amplitude of tactile startle reflex, but significantly disrupted H cross-modal PPI in CIN-d males, as compared with both Ctrl males and CIN-d females. I Bright ambient light elicited a significant enhancement in marble-burying behaviors in male, but not female, CIN-d mice. The same stressor exacerbated anxiety-like responses in males in the elevated plus maze, as shown by the significant reduction of J open-arm time, K open-arm entries, and L exploratory head dips. All animals were exposed to either SC (A–H) or bright environmental light (I–L). Data were analyzed with two-way ANOVA designs, except for PPIs, which were analyzed by three-way, repeated-measure ANOVAs. *P < 0.05; ***P < 0.001 for comparisons between CIN-d male vs Ctrl male; #P < 0.05; ##P < 0.01; ###P < 0.001 for comparisons between CIN-d females vs CIN-d males. All data are shown as means ± SEM. n = 8/group. For further details, see text and Supplementary Table 1B.
To exclude the potential influence of the circadian rhythm on these behavioral responses, the effects of stress were also tested in a separate cohort of mice during the light phase (Supplementary Fig. 4 and Supplementary Table 1G). These experiments confirmed a major increase in tic-related behaviors (grooming, digging, and sporadic jerks) in spatially confined CIN-d males in comparison with all other groups (n = 8/group) (Supplementary Fig. 4A–C). Conversely, eye blinking frequency and acoustic startle amplitude were increased in both male and female CIN-d mice (Supplementary Fig. 4D, E). Finally, CIN-d males exhibited significant PPI deficits after spatial confinement (Supplementary Fig. 4F). In line with our previous findings, CIN-d males in the light phase displayed significant elevations of marble-burying responses when exposed to a brightly lit environment, but not under regular ambient illumination (Supplementary Fig. 4G, H).
Tic-like behaviors are sensitive to TS therapies in CIN-d mice
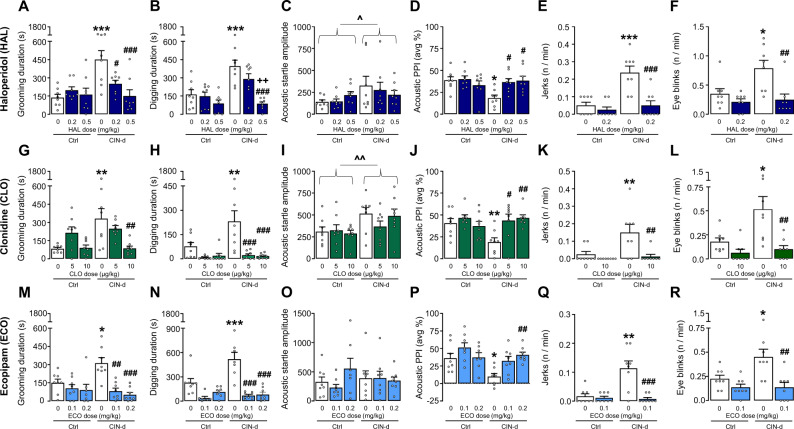
To verify the predictive validity of the observed TS-like behavioral alterations in CIN-d mice, we tested the effects of several drugs known to reduce the severity of tics and TS-associated symptoms, including the benchmark therapies haloperidol (HAL) and clonidine (CLO) and the potent D1 receptor antagonist ecopipam (ECO), which was recently shown to reduce tic severity in TS [41]. Testing was performed in CIN-d male mice and controls under conditions of spatial confinement (n = 8/group) (Fig. 3 and Supplementary Table 1C). The prototypical antipsychotic HAL (0.2–0.5 mg/kg, IP, injected 15 min before testing) reduced stress-elicited increases in grooming (Fig. 3A) [CIN-d × HAL interaction: F(2,42) = 6.80, P = 0.003; partial η2 = 0.24] and digging stereotypies (Fig. 3B). In addition, this drug reversed the PPI deficits induced by spatial confinement in CIN-d mice (Fig. 3D) [F(2,42) = 4.71, P = 0.01; partial η2 = 0.20], without affecting startle amplitude (Fig. 3C). HAL (0.2 mg/kg) also rescued other fine motor behavioral abnormalities, including sporadic head jerks (Fig. 3E) [F(1,28) = 9.62, P = 0.004; partial η2 = 0.26] and eye blinks (Fig. 3F) [F(1,28) = 4.23, P = 0.04; partial η2 = 0.13]. Very similar effects on these behavioral responses were elicited by the α2 adrenergic agonist CLO (5–10 µg/kg, IP, injected 15 min before testing) (Fig. 3G–L), and the D1 receptor blockers ECO (0.1–0.2 mg/kg, IP, injected 30 min before testing) (Fig. 3M–R) and SCH23390 (0.2–0.5 mg/kg, IP, injected 15 min before testing) (Supplementary Fig. 5 and Supplementary Table 1H). Notably, the effective doses of HAL, CLO, and ECO did not affect locomotor activity in control mice (Supplementary Fig. 6 and Supplementary Table 1I), suggesting that the observed effects on TS-relevant responses were not due to experimental artifacts secondary to hypolocomotion.
Fig. 3. Effects of Tourette syndrome (TS) treatments on repetitive behaviors, as well as prepulse inhibition (PPI) deficits in CIN-depleted (CIN-d) male mice exposed to spatial confinement (SC).
The antipsychotic haloperidol (HAL; 0.2-0.5 mg/kg, IP, injected 15 min before testing) reduced SC-induced increases in A total grooming and B digging durations. While CIN-d mice also exhibited increased C acoustic startle reflex amplitude, this index was not affected by HAL; this drug reversed D PPI deficits in SC-exposed CIN-d mice. HAL (0.2 mg/kg) also normalized the frequency of E head jerks and F eye blinks. Clonidine (CLO; 5–10 µg/kg, injected 15 min before testing) elicited similar effects, as shown by the analyses of G total grooming duration, H total digging duration, I acoustic startle amplitude, J acoustic PPI, K jerk frequency and L eye blink frequency. Ecopipam (ECO; 0.1-0.2 mg/kg, IP, injected 30 min before testing) also reversed the detrimental effects of SC on M grooming and N digging durations and P rescued PPI deficits in CIN-d mice, without affecting O startle responses. This drug also reversed increases in the frequency of Q jerks and R eye blinks. All data were analyzed with two-way ANOVAs. ^P < 0.05; ^^P < 0.01, main effect for CIN-d vs control (Ctrl) mice. *P < 0.05; **P < 0.01; ***P < 0.001 for post-hoc comparisons between treatment vehicles (represented by white bars)-treated CIN-d and Ctrl mice. #P < 0.05; ##P < 0.01; ###P < 0.001 for comparisons between CIN-d mice injected with either treatment or its vehicle; ++P < 0.01 for post-hoc comparisons between CIN-d mice treated with 0.2 and 0.5 mg/kg of HAL. All data refer to SC‐exposed CIN-d and Ctrl male mice. Treatment doses are indicated under colored bars and refer to IP administrations. All data are shown as means ± SEM. n = 8/group. For further details, see text and Supplementary Table 1C.
AP elicits and worsens TS-related responses in freely moving CIN-d mice
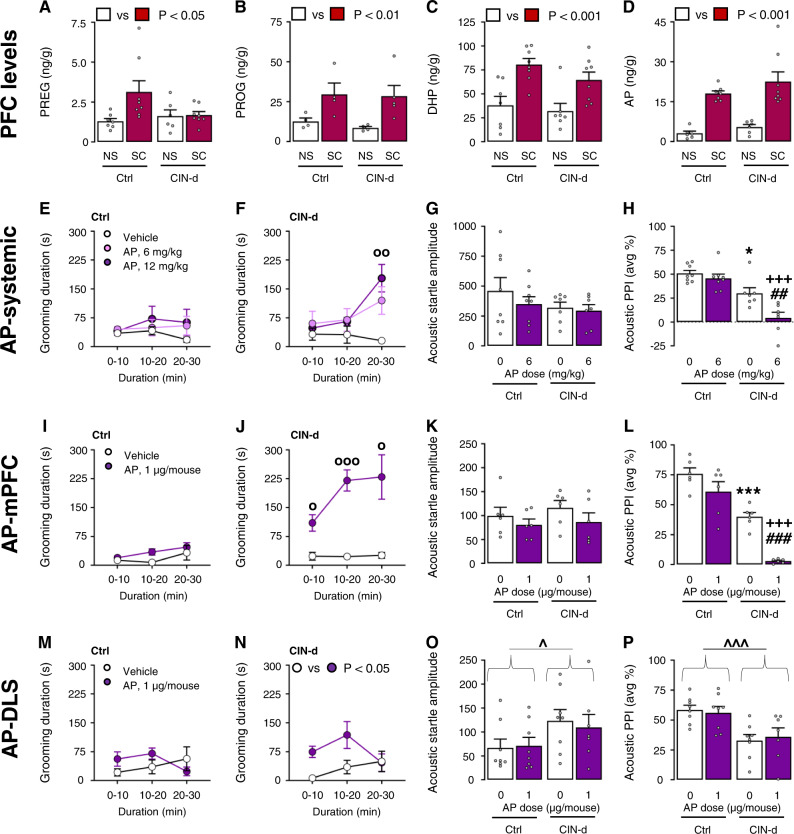
We previously showed that spatial confinement led to an increase of AP in the PFC of D1CT-7 mice and their BALB/c controls [26]. However, to verify whether the same manipulation may elicit similar neurochemical effects in CIN-d mice and their controls (which have a C57BL/6 background), we measured the PFC levels of AP and its precursors pregnenolone, progesterone, and 5α-dihydroprogesterone in these experimental groups. These experiments confirmed that spatial confinement markedly enhanced the PFC content of these steroids in both CIN-d mice and their controls (Fig. 4A–D and Supplementary Table 1D). Building on these results, we then tested whether AP was sufficient to produce or exacerbate TS-relevant behaviors in freely moving CIN-d male mice in comparison with controls (Fig. 4E). Systemic AP injections (6–12 mg/kg, IP, 5 min before testing) markedly increased grooming behavior in CIN-d after 20–30′ from administration, but not control mice (Fig. 4E, F) [Time × AP interaction: F(4,42) = 2.77, P = 0.04; partial η2 = 0.21]. We then tested the effects of AP on acoustic PPI at 6 mg/kg (IP) - a low systemic dose that does not intrinsically impair sensorimotor gating in C57BL/6 mice [42]. AP did not change startle amplitude in either group of mice (Fig. 4G); however, this drug did produce a significant additional PPI deficit in CIN-d animals (Fig. 4H) [F(1,26) = 4.50, P = 0.04; partial η2 = 0.15]. The analysis of tactile startle and cross-modal PPI revealed very similar results of AP (Supplementary Fig. 7 and Supplementary Table 1J). To ascertain the neuroanatomical basis of these effects, we tested the impact of local AP injections (1 µg/µl/mouse) either in the medial (m)PFC (Fig. 4I–L) or the dorsolateral striatum (DLS) (Fig. 4M–P), two areas implicated in tic pathophysiology. In contrast with controls (Fig. 4I), cortical AP administration significantly increased time spent grooming in CIN-d mice with a peak during 10–30′ from administration (Fig. 4J) [Time × AP interaction: F(2,28) = 3.64, P = 0.04; partial η2 = 0.21]; cortical AP also exacerbated spontaneous PPI deficits [F(1,20) = 4.70, P = 0.04; partial η2 = 0.19], without affecting startle amplitude (Fig. 4K, L). In contrast, intra-DLS administration of AP only elicited a mild increase of grooming responses in CIN-d mice at 10–20′ from administration (Fig. 4M, N) but did not modify either the startle reflex or PPI (Fig. 4O–P).
Fig. 4. Allopregnanolone (AP) increased Tourette syndrome- (TS) relevant responses in CIN-depleted (CIN-d) but not control (Ctrl) male mice.
Spatial confinement (SC; 40 min) increased the levels of A pregnenolone (PREG); B progesterone (PROG); C 5α-dihydroprogesterone (DHP); and D AP in the prefrontal cortex (PFC) of CIN-d and Ctrl male mice. While systemic AP injections (6-12 mg/kg, IP, 5 min before testing) did not alter E grooming duration in Ctrl mice, it increased this response F in CIN-d mice after 20 min from its administration. AP elicited no significant effect on G startle amplitude in either Ctrl or CIN-d mice but did exacerbate H PPI deficits in CIN-d mice. Similarly, AP administration into the medial prefrontal cortex (mPFC) (1 µg/µl/mouse) failed to increase grooming duration in I Ctrl mice but markedly enhanced this response J in CIN-d mice. AP failed to modify startle response K but exacerbated PPI impairments L. Administration of AP into the dorsolateral striatum (DLS) increased moderately total grooming duration in N CIN-d, but not control (M) mice. While CIN-d mice showed increased acoustic startle amplitude (O) and reduced PPI (P), these effects were not modified by AP. OP < 0.05; OOP < 0.01; OOOP < 0.001 for post-hoc comparisons between the effects of AP and vehicle at the same time from administration. ^P < 0.05; ^^^P < 0.001 for main effect of CIN-d. *P < 0.05; ***P < 0.001 for post-hoc comparisons between vehicle (represented by white bars)-treated CIN-d and Ctrl mice. ##P < 0.01; ###P < 0.001 for comparisons between CIN-d mice injected with AP and its vehicle; +++P < 0.001 for post-hoc comparisons between Ctrl and CIN-d mice treated with AP. Grooming data were analyzed by two-way, repeated-measure ANOVA designs. Acoustic startle and PPI data were analyzed with two-way ANOVAs. All data refer to freely moving CIN-d and Ctrl male mice. AP doses are reported under colored bars. All data are shown as means ± SEM. n = 6-8/group. For further details, see text and Supplementary Table 1D.
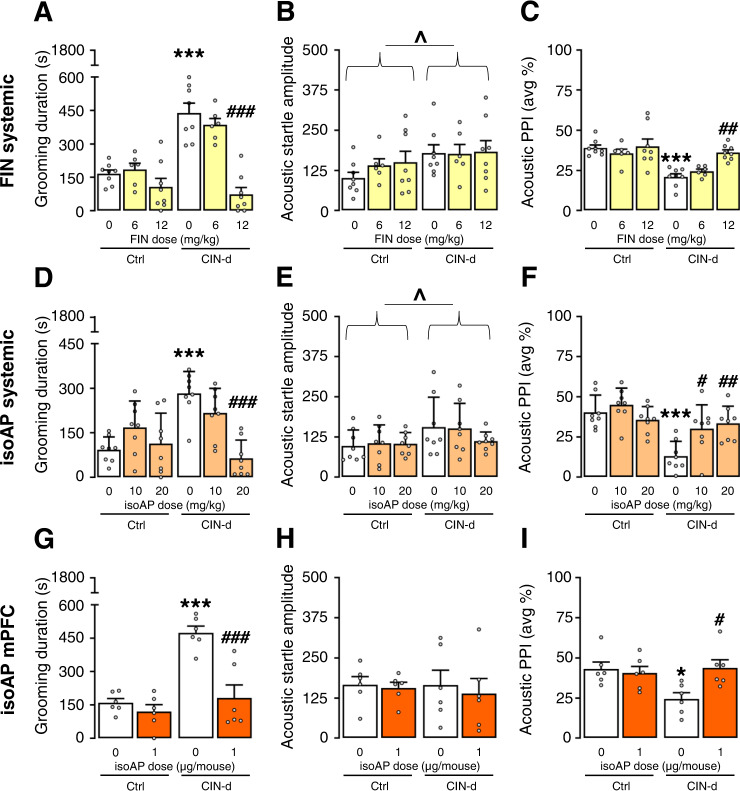
Opposing AP synthesis or signaling reduces the effects of spatial confinement stress in CIN-d mice
To verify whether AP is necessary for the greater sensitivity of CIN-d mice to acute stress, we tested the impact of the AP synthesis inhibitor finasteride (FIN, 6–12 mg/kg, IP, 15 min before testing) on the behavioral effects of spatial confinement (Fig. 5 and Supplementary Table 1E). FIN dose-dependently blocked the stress-induced increase in grooming (Fig. 5A) [F(2,38) = 12.69, P < 0.0001; partial η2 = 0.40] and digging (Supplementary Fig. 8A and Supplementary Table 1K) and reversed PPI deficits (Fig. 5C) [F(2,38) = 3.94, P = 0.03; partial η2 = 0.17] in CIN-d mice without affecting startle amplitude (Fig. 5B).
Fig. 5. Blocking allopregnanolone (AP) synthesis and signaling normalized the adverse impact of spatial confinement (SC) in CIN-depleted (CIN-d) mice.
The inhibitor of AP synthesis finasteride (FIN; 6 and 12 mg/kg, IP) dose-dependently countered SC-induced elevations in grooming duration (A) and PPI deficits in CIN-d mice (C), without affecting startle amplitude (B). Systemic administration of the AP antagonist isoAP (10 and 20 mg/kg, IP) also opposed the increase in grooming induced by SC (D). As expected, CIN-d led to the enhancement of startle amplitude (E), but isoAP did not affect this parameter. However, isoAP (10-20 mg/kg, IP, 10 min before testing) reversed PPI deficits in CIN-d mice (F). IsoAP infusion into the medial prefrontal cortex (mPFC) (1 μg/μl/mouse) also reversed the negative effects of SC on grooming (G). While no significant differences were found in startle amplitude (H), local isoAP infusions normalized PPI in CIN-d mice (I). All data were analyzed by two-way ANOVAs. ^P < 0.05; main effects for CIN-d. *P < 0.05; ***P < 0.001 for post-hoc comparisons between CIN-d and Ctrl mice treated with vehicle (represented by white bars). #P < 0.05; ##P < 0.01; ###P < 0.001 for comparisons between CIN-d mice injected with either the treatment or its vehicle. All data refer to SC‐exposed CIN-d and Ctrl male mice. Treatment doses are indicated under colored bars. All data are shown as means ± SEM. n = 6–8/group. For further details, see text and Supplementary Table 1E.
We then tested the effects of systemic administration of the AP antagonist isoAP and found that this drug also blocked the effects of spatial confinement on grooming (Fig. 5D) [F(2,42) = 9.57, P = 0.0004; partial η2 = 0.31] and on digging (Supplementary Fig. 8B and Supplementary Table 1K). Furthermore, systemic isoAP reversed the PPI deficits in CIN-d mice (10–20 mg/kg, IP, 10 min before testing; Fig. 5F) [F(2,42) = 5.39, P = 0.008; partial η2 = 0.20]. While startle amplitude was increased by partial CIN depletion, it was not affected by isoAP (Fig. 5E). Very similar effects were observed after isoAP infusion directly into the mPFC (1 μg/μl/mouse). Indeed, this treatment reversed the negative effects of spatial confinement on grooming (Fig. 5G) [F(1, 20) = 11.50, P = 0.003; partial η2 = 0.36] and PPI (Fig. 5I) [F(1,20) = 6.27, P = 0.02; partial η2 = 0.24], without any significant effects on startle amplitude (Fig. 5H).
Discussion
In the present study, we developed a model of early-life dorsal striatal CIN depletion using the strategy previously applied by Xu et al. [20] to generate a targeted loss of striatal CINs in adult mice. In males, early-life partial striatal CIN depletion resulted in baseline PPI deficits akin to the sensorimotor gating impairments observed in TS [43]. While CIN-d mice did not display any alterations in baseline self-grooming or digging activity, males showed a marked increase in these stereotypies in response to spatial confinement – a mild acute stressor that had no overt effects in control mice [25]. CIN-d males also exhibited elevated marble-burying, a reflection of perseverative digging [44], but only under bright light, a well-documented environmental stressor in laboratory rodents [45]. Notably, similar phenotypic differences between CIN-d and control males were documented in both the dark and light phases, ruling out potential influences of the circadian rhythms in the observed behavioral responses. Although grooming and digging stereotypies are typical responses to environmental stress in experimental animals [46, 47], CIN-d mice did not show any specific enhancement of anxiety-like behavior in the elevated plus maze, suggesting that their stereotyped responses may reflect a greater propensity to enact repetitive behaviors in response to stress, rather than a higher sensitivity to stress itself. This interpretation is supported by previous work suggesting that striatal CIN depletion impairs behavioral flexibility. For example, several authors documented that inactivation of striatal CINs or disruption of their connectivity impairs reversal and set-shifting, but not baseline, learning [48–51]. Furthermore, striatal CIN depletion produces perseverative social responses [52], suggesting that striatal CINs enable the extinction of adaptive and salience-driven motor sequences.
The finding of stress-sensitive stereotypies in CIN-d mice supports the contention that these motor responses recapitulate pathophysiological processes relevant to TS. Indeed, stereotypies share both neurobiological mechanisms and phenomenological characteristics with tics, including their sensitivity to contextual stressors [53]. From this perspective, our findings highlight the model of early-life striatal CIN depletion as a tool with high face and construct validity, optimally suited to studying the neurobiological foundations of TS and comorbid disorders. Notably, the value of these models with respect to TS is further supported by the sexual dimorphism of its behavioral manifestations, which parallels the male preponderance of TS [54]. Furthermore, the high predictive validity for TS was confirmed by our finding that the behavioral abnormalities featured by CIN-d by mice were reversed by haloperidol and clonidine, two benchmark therapies for tic disorders, and ecopipam, which has been recently highlighted as an effective TS treatment [41]. Although it should be noted that these behavioral effects were observed after acute treatment (while all these drugs elicit therapeutic effects only after chronic administration in patients), these results indicate that CIN-d mice may also serve as a useful platform to screen new therapeutics for tic disorders.
We have further shown that the effects of an acute mild stressor on CIN-d mice reflect increased levels of the neurosteroid AP in the PFC. Systemic and intra-PFC administration of AP elicited behavioral responses akin to those of acute stress in CIN-d mice and blocking AP synthesis (via FIN) or signaling (via isoAP) reversed the effects of stress in CIN-d mice without altering the behavior of control mice. These findings confirm and extend previous evidence on the key role of AP in mediating the adverse behavioral effects on TS-relevant behaviors in D1CT-7 mice, a classical model of TS with high face and predictive validity [26]. Furthermore, our data align strongly with previous results on the efficacy of FIN and isoAP in reducing stereotyped behaviors and PPI deficits across several animal models, including D1CT-7 mice [26, 29, 42, 55, 56]. Notably, the effects of FIN and isoAP were comparable to those elicited by haloperidol, suggesting that the negative modulation of AP functions may elicit similar therapeutic effects. In line with this idea, we previously showed, in a proof-of-concept open-label trial, that FIN reduced the severity and frequency of tics and compulsions in adult male TS patients [57]. A placebo-controlled trial of isoAP in TS is ongoing (Clinical Trial NCT05434546).
We also showed that spatial confinement markedly increased AP content in this region in both CIN-d and control mice. These data are in line with previous findings indicating that this and other acute stressors enhance AP levels in the PFC [22, 26, 42]. The best-defined mechanism of action of AP is the positive allosteric modulation of GABAA receptors [27, 58, 59] through the activation of a specific site in the α subunit of these channels [60, 61]. At higher (micromolar) concentrations, AP acts as a GABAA receptor agonist by binding to a second site located at the interface between α and β subunits [61]. In line with these mechanisms, the detrimental effects of AP on PPI were opposed by isoAP, a selective AP antagonist [30].
Ample evidence shows that the PFC sends direct efferent projections to the striatum [62], which subserve inhibitory behavioral control [63]. Local GABAergic inhibition of projection cells in the mPFC may disinhibit perseverative responses in the context of other local striatal predisposing factors, such as a partial loss of CINs. Given that AP activates both extrasynaptic and synaptic GABAA receptors and that its affinity for different targets is influenced by the specific subunit composition of these ionotropic channels [64], it is likely that sudden elevations of AP in the mPFC can alter the balance between excitatory and inhibitory neural activity in this region and in its projections to the striatum. From a translational perspective, it is worth noting that activation of the PFC has been extensively linked to tic suppression in TS [65–67], and that preliminary evidence suggests that the main adverse effect of acute stress in TS may lie in the disruption of tic suppression [68] (see [69] for a detailed discussion of this potential mechanism).
The observation of spatially confined mice allowed for the characterization of other spontaneous, sporadic fine movements, such as eyeblink and head jerks, that could not be monitored in freely moving conditions. Notably, CIN-d mice exhibited higher frequencies of both responses in comparison with controls. Given that both motor manifestations bear striking isomorphism to simple tics and are reduced by haloperidol, it is tempting to speculate that these behaviors reflect TS-related motor responses. However, given that these responses could only be captured under spatial confinement, it remains unclear whether AP plays a role in these alterations.
In contrast with mice that underwent DSL CIN depletion in adulthood [20], our juvenile CIN depletion mice show spontaneous PPI deficits in males but not in females. This discrepancy may reflect a greater sensitivity of younger male mice to the impact of partial CIN depletion on the regulation of sensorimotor gating. Alternatively, PPI deficits may be driven by the marginal CIN depletion in the ventral striatum, given the key role of the nucleus accumbens in the modulation of PPI [70, 71].
Our analyses revealed that, although the degree of CIN depletion in males and females was statistically comparable, the number of CINs in the dorsal and ventral striatum was respectively lower and higher in females. To the best of our knowledge, this is the first evidence indicating a baseline difference in CIN number in the striatum. While the neurobiological underpinnings of this sexual dimorphism remain unknown, it is possible that such differences result in compensatory functional modifications, which may underlie the lower sensitivity of females to the effects of partial CIN depletion in the dorsal striatum. On this basis, future studies are warranted to understand whether and how differences in CIN number in the striatum may account for the male preponderance of TS in children.
Several limitations of the study should be recognized. First, although our results point to a key role of AP in the regulation of TS-like responses in CIN-d mice, we cannot exclude the possibility that other neurosteroids also participate in the adverse behavioral effects of stress. Further investigations will need to focus on other neurosteroids increased by stress, such as tetrahydrodeoxycorticosterone, or androgenic neuroactive steroids, as well as the role of the GABAA receptor and other AP-sensitive receptors. Second, while our results indicate that AP mediates its effects through the activation of GABAA receptors in the PFC, the specific downstream mechanisms whereby this process interferes with tic execution remain unclear. Despite these caveats, our study provides support for the hypothesis that AP plays an important role in the modulation of TS-related phenotypes, especially in the context of stress, and that isoAP and FIN may be useful therapeutic tools for the reduction of tics. Given the complementary value of CIN-d and D1CT-7 mice in capturing distinct aspects of TS phenomenology and pathophysiology [53, 72], our convergent results in these two studies [26] provide synergistic support for this mechanism in the regulation of tic fluctuations in TS. Future studies will be essential to confirm these findings in TS patients and to further explore the therapeutic potential of neurosteroid-targeting therapies in tic disorders.
Supplementary information
Acknowledgements
We would like to thank Dr. Caterina Branca for her precious editorial assistance and suggestions.
Author contributions
RC: Behavioral and pharmacological investigation, formal analysis, Writing – original draft, writing – review & editing. MVZ: Immunohistochemical investigation, quantification and images acquisition, formal analysis. LS: Steroid measurements. KOO: Behavioral investigation. CJA: Writing – review & editing. DF: Immunohistochemical investigation, Quantification and Images acquisition. PN: Writing – review & editing. GP: Data curation and analysis. CP: Conceptualization, Methodology, Data curation, Writing – review & editing, Funding acquisition. MB: Conceptualization, methodology, data curation, writing – original draft, writing – review & editing, funding acquisition.
Funding
This study was supported by the NIH grants R21 NS108722 (to MB and CP) and R21 NS125654 (to MB).
Competing interests
PN is CEO and CMO for Asarina Pharma, has a 1.2% equity stake in Asarina Pharma, and has filed patents for the use of sepranolone in the treatment of Tourette syndrome, obsessive-compulsive disorder, and pathological gambling. GP is a paid consultant to PureTech Health, GABA Therapeutics, and NeuroTrauma Sciences. He has a pending patent application on N-palmitoylethanolamine (PEA) and peroxisome proliferator-activated receptor alpha (PPAR-α) agonists, and another one on allopregnanolone analogs US11266663B2 granted on March 8, 2022 in the treatment of neuropsychiatric disorders. CP consults for Biohaven Pharmaceuticals, Ceruvia Lifesciences, Transcend Therapeutics, Freedom Biosciences, Nobilis Therapeutics, and UCB Biopharma and receives research funding from Biohaven, Transcend, and Freedom. He has filed patents for neurofeedback and psychedelics in the treatment of obsessive-compulsive disorder, unrelated to the current work. MB consults for Asarina Pharmaceuticals and receives research funding from Asarina and Lundbeck Pharmaceuticals. The other authors declare no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christopher Pittenger, Marco Bortolato.
Contributor Information
Christopher Pittenger, Email: christopher.pittenger@yale.edu.
Marco Bortolato, Email: marco.bortolato@utah.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-023-01603-6.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed., text rev. Washington: American Psychiatric Association; 2022.
- 2.Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325–33. doi: 10.1001/jamapsychiatry.2014.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizzo R, Gulisano M, Martino D, Robertson MM. Gilles de la Tourette Syndrome, depression, depressive illness, and correlates in a child and adolescent population. J Child Adolesc Psychopharmacol. 2017;27:243–9. doi: 10.1089/cap.2016.0120. [DOI] [PubMed] [Google Scholar]
- 4.Quezada J, Coffman KA. Current approaches and new developments in the pharmacological management of Tourette syndrome. CNS Drugs. 2018;32:33–45. doi: 10.1007/s40263-017-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kompoliti K, Goetz CG, Morrissey M, Leurgans S. Gilles de la Tourette syndrome: patient’s knowledge and concern of adverse effects. Mov Disord. 2006;21:248–52. doi: 10.1002/mds.20680. [DOI] [PubMed] [Google Scholar]
- 6.Jerrell JM, McIntyre RS. Adverse events in children and adolescents treated with antipsychotic medications. Hum Psychopharmacol. 2008;23:283–90. doi: 10.1002/hup.932. [DOI] [PubMed] [Google Scholar]
- 7.Szejko N, Lombroso A, Bloch MH, Landeros-Weisenberger A, Leckman JF. Refractory Gilles de la Tourette syndrome-many pieces that define the puzzle. Front Neurol. 2020;18:589511. doi: 10.3389/fneur.2020.589511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankovic J. Diagnosis and classification of tics and Tourette syndrome. Adv Neurol. 1992;58:7–14. [PubMed] [Google Scholar]
- 9.Bornstein RA, Stefl ME, Hammond L. A survey of Tourette syndrome patients and their families: the 1987 Ohio Tourette Survey. J Neuropsychiatry Clin Neurosci. 1990;2:275–81. doi: 10.1176/jnp.2.3.275. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg T, Shmuel-Baruch S, Horesh N, Apter A. Life events and Tourette syndrome. Compr Psychiatry. 2013;54:467–73. doi: 10.1016/j.comppsych.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Lin H, Katsovich L, Ghebremichael M, Findley DB, Grantz H, Lombroso PJ, et al. Psychosocial stress predicts future symptom severities in children and adolescents with Tourette syndrome and/or obsessive-compulsive disorder. J Child Psychol Psychiatry. 2007;48:157–66. doi: 10.1111/j.1469-7610.2006.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findley DB, Leckman JF, Katsovich L, Lin H, Zhang H, Grantz H, et al. Development of the Yale Children’s Global Stress Index (YCGSI) and its application in children and adolescents with Tourette’s syndrome and obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 2003;42:450–7. doi: 10.1097/01.CHI.0000046816.95464.EF. [DOI] [PubMed] [Google Scholar]
- 13.Hoekstra PJ, Steenhuis MP, Kallenberg CG, Minderaa RB. Association of small life events with self reports of tic severity in pediatric and adult tic disorder patients: a prospective longitudinal study. J Clin Psychiatry. 2004;65:426–31. doi: 10.4088/jcp.v65n0320. [DOI] [PubMed] [Google Scholar]
- 14.Godar SC, Bortolato M. What makes you tic? Translational approaches to study the role of stress and contextual triggers in Tourette syndrome. Neurosci Biobehav Rev. 2017;76:123–33. doi: 10.1016/j.neubiorev.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buse J, Enghardt S, Kirschbaum C, Ehrlich S, Roessner V. Tic frequency decreases during short-term psychosocial stress - an experimental study on children with Tic disorders. Front Psychiatry. 2016;7:84. doi: 10.3389/fpsyt.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci USA. 2005;102:13307–12. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lennington JB, Coppola G, Kataoka-Sasaki Y, Fernandez TV, Palejev D, Li Y, et al. Transcriptome analysis of the human striatum in Tourette syndrome. Biol Psychiatry. 2016;79:372–82. doi: 10.1016/j.biopsych.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–82. doi: 10.1016/j.tins.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Xu M, Kobets A, Du JC, Lennington J, Li L, Banasr M, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci USA. 2015;112:893–8. doi: 10.1073/pnas.1419533112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bortolato M, Frau R, Godar SC, Mosher LJ, Paba S, Marrosu F, et al. The implication of neuroactive steroids in Tourette’s syndrome pathogenesis: a role for 5α-reductase? J Neuroendocrinol. 2013;25:1196–208. doi: 10.1111/jne.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma- aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125–39. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunn BG, Cunningham L, Mitchell SG, Swinny JD, Lambert JJ, Belelli D. GABAA receptor-acting neurosteroids: a role in the development and regulation of the stress response. Front Neuroendocrinol. 2015;36:28–48. doi: 10.1016/j.yfrne.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godar SC, Mosher LJ, Strathman HJ, Gochi AM, Jones CM, Fowler SC, et al. The D1CT-7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. Br J Pharmacol. 2016;173:2111–21. doi: 10.1111/bph.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosher LJ, Godar SC, Nelson M, Fowler SC, Pinna G, Bortolato M. Allopregnanolone mediates the exacerbation of Tourette-like responses by acute stress in mouse models. Sci Rep. 2017;7:3348. doi: 10.1038/s41598-017-03649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 28.Puia G, Santi MR, Vicini S, Pritchett DB, Purdy RH, Paul SM, et al. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–65. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 29.Lundgren P, Strömberg J, Bäckström T, Wang M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3beta-hydroxy-5alpha-pregnan-20- one (isoallopregnanolone) Brain Res. 2003;982:45–53. doi: 10.1016/s0006-8993(03)02939-1. [DOI] [PubMed] [Google Scholar]
- 30.Bengtsson SK, Nyberg S, Hedström H, Zingmark E, Jonsson B, Bäckström T, et al. Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans. Psychoneuroendocrinology. 2015;52:22–31. doi: 10.1016/j.psyneuen.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Cadeddu R, Bäckström T, Floris G, Nordkild P, Segerdahl M, Bortolato M. Isoallopregnanolone reduces tic-like behaviours in the D1CT-7 mouse model of Tourette syndrome. J Neuroendocrinol. 2020;32:e12754. doi: 10.1111/jne.12754. [DOI] [PubMed] [Google Scholar]
- 32.Bortolato M, Frau R, Orrù M, Piras AP, Fà M, Tuveri A, et al. Activation of GABA(B) receptors reverses spontaneous gating deficits in juvenile DBA/2J mice. Psychopharmacology. 2007;194:361–9. doi: 10.1007/s00213-007-0845-5. [DOI] [PubMed] [Google Scholar]
- 33.Godar SC, Bortolato M, Frau R, Dousti M, Chen K, Shih JC. Maladaptive defensive behaviours in monoamine oxidase A-deficient mice. Int J Neuropsychopharmacol. 2011;14:1195–207. doi: 10.1017/S1461145710001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wyatt LR, Godar SC, Khoja S, Jakowec MW, Alkana RL, Bortolato M, et al. Sociocommunicative and sensorimotor impairments in male P2X4-deficient mice. Neuropsychopharmacology. 2013;38:1993–2002. doi: 10.1038/npp.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bortolato M, Chen K, Godar SC, Chen G, Wu W, Rebrin I, et al. Social deficits and perseverative behaviors, but not overt aggression, in MAO-A hypomorphic mice. Neuropsychopharmacology. 2011;36:2674–88. doi: 10.1038/npp.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 6th ed. Amsterdam, The Netherlands: Academic; 2007.
- 37.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci USA. 1996;93:12599–604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci USA. 2003;100:2035–40. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82.. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapanelli M, Frick LR, Xu M, Groman SM, Jindachomthong K, Tamamaki N, et al. Targeted interneuron depletion in the dorsal striatum produces autism-like behavioral abnormalities in male but not female mice. Biol Psychiatry. 2017;82:194–203. doi: 10.1016/j.biopsych.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilbert DL, Murphy TK, Jankovic J, Budman CL, Black KJ, Kurlan RM, et al. Ecopipam, a D1 receptor antagonist, for treatment of tourette syndrome in children: a randomized, placebo-controlled crossover study. Mov Disord. 2018;33:1272–80. doi: 10.1002/mds.27457. [DOI] [PubMed] [Google Scholar]
- 42.Cadeddu R, Mosher LK, Nordkild P, Gaikwad N, Ratto GM, Scheggi S, et al. Acute stress impairs sensorimotor gating via the neurosteroid allopregnanolone in the prefrontal cortex. Neurobiol Stress. 2022;21:100489. doi: 10.1016/j.ynstr.2022.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette’s syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 44.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 2009;204:361–73. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patchev VK, Patchev AV. Experimental models of stress. Dialogues Clin Neurosci. 2006;8:417–32. doi: 10.31887/DCNS.2006.8.4/vpatchev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broekkamp CL, Rijk HW, Joly-Gelouin D, Lloyd KL. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur J Pharmacol. 1986;126:223–9. doi: 10.1016/0014-2999(86)90051-8. [DOI] [PubMed] [Google Scholar]
- 47.Moody TW, Merali Z, Crawley JN. The effects of anxiolytics and other agents on rat grooming behavior. Ann N Y Acad Sci. 1988;525:281–90. doi: 10.1111/j.1749-6632.1988.tb38613.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–8. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradfield LA, Bertran-Gonzalez J, Chieng B, Balleine BW. The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron. 2013;79:153–66. doi: 10.1016/j.neuron.2013.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aoki S, Liu AW, Zucca A, Zucca S, Wickens JR. Role of striatal cholinergic interneurons in set-shifting in the rat. J Neurosci. 2015;35:9424–31. doi: 10.1523/JNEUROSCI.0490-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beccaria JP, Pretell Annan CA, Keifman E, Murer MG, Belforte JE. Striatal cholinergic interneurons are required for contending strategy selection while solving spatial navigation problems. J Neurosci. 2022;42:1303–15. doi: 10.1523/JNEUROSCI.1130-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martos YV, Braz BY, Beccaria JP, Murer MG, Belforte JE. Compulsive social behavior emerges after selective ablation of striatal cholinergic interneurons. J Neurosci. 2017;37:2849–58. doi: 10.1523/JNEUROSCI.3460-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bortolato M, Pittenger C. Modeling tics in rodents: conceptual challenges and paths forward. J Neurosci Methods. 2017;292:12–19. doi: 10.1016/j.jneumeth.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freeman RD. Diagnostic criteria for Tourette’s disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:209–10. doi: 10.1097/01.chi.0000150617.35370.47. [DOI] [PubMed] [Google Scholar]
- 55.Devoto P, Frau R, Bini V, Pillolla G, Saba P, Flore G, et al. Inhibition of 5α-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2012;37:1630–45. doi: 10.1016/j.psyneuen.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frau R, Mosher LJ, Bini V, Pillolla G, Pes R, Saba P, et al. The neurosteroidogenic enzyme 5α-reductase modulates the role of D1 dopamine receptors in rat sensorimotor gating. Psychoneuroendocrinology. 2016;63:59–67. doi: 10.1016/j.psyneuen.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord. 2011;26:2146–7. doi: 10.1002/mds.23810. [DOI] [PubMed] [Google Scholar]
- 58.Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- 59.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–68. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 60.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 61.Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology. 2009;56:149–54. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 62.Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26:8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terra H, Bruinsma B, de Kloet SF, van der Roest M, Pattij T, Mansvelder HD. Prefrontal cortical projection neurons targeting dorsomedial striatum control behavioral inhibition. Curr Biol. 2020;30:4188–4200.e5. doi: 10.1016/j.cub.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 64.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 65.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55:326–33. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 66.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. Functional magnetic resonance imaging of tics and tic suppression in Gilles de la Tourette syndrome. World J Biol Psychiatry. 2009;10:567–70. doi: 10.1080/15622970802118356. [DOI] [PubMed] [Google Scholar]
- 67.Rae CL, Parkinson J, Betka S, Gouldvan Praag CD, Bouyagoub S, Polyanska L, et al. Amplified engagement of prefrontal cortex during control of voluntary action in Tourette syndrome. Brain Commun. 2020;2:fcaa199. doi: 10.1093/braincomms/fcaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Conelea CA, Woods DW, Brandt BC. The impact of a stress induction task on tic frequencies in youth with Tourette Syndrome. Behav Res Ther. 2011;49:492–7. doi: 10.1016/j.brat.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Bortolato M, Coffey BJ, Gabbay V, Scheggi S. Allopregnanolone: the missing link to explain the effects of stress on tic exacerbation? J Neuroendocrinol. 2022;34:e13022. doi: 10.1111/jne.13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wan FJ, Geyer MA, Swerdlow NR. Presynaptic dopamine-glutamate interactions in the nucleus accumbens regulate sensorimotor gating. Psychopharmacology. 1995;120:433–41. doi: 10.1007/BF02245815. [DOI] [PubMed] [Google Scholar]
- 71.Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res. 1996;722:168–76. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 72.Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods. 2014;238:54–69. doi: 10.1016/j.jneumeth.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.