Table 1.
1H-Imidazo[4,5-c]quinolin-4-amine Derivatives Synthesized and Effects on Agonist-Induced A3AR Activation
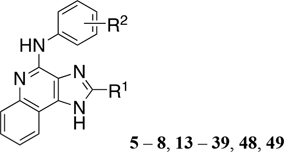
| |||
|---|---|---|---|
| Compound | R2 | R1 | Emaxa(%) |
| 2-alkyl and 2-cycloalkyl derivatives | |||
| 13 | 3,4-Cl2 |
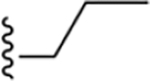
|
153 ± 12* |
| 14 | 3,4-Cl2 |
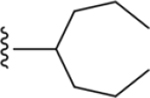
|
216 ± 12* |
| 15 | 3,4-Cl2 |
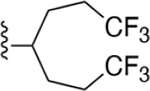
|
159 ±13* |
| 16 | 3,4-Cl2 |
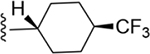
|
150 ± 5* |
| 17 | 3,4-Cl2 |
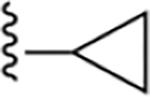
|
111 ± 16 |
| 5 | 3,4-Cl2 |
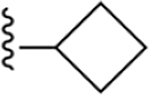
|
109 ± 3 |
| 6 | 3,4-Cl2 |
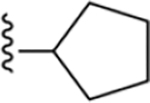
|
142 ± 5* |
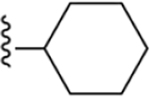
| 7 | 3,4-Cl2 |
|
225 ± 10* |
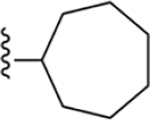
| 8 | 3,4-Cl2 |
|
175 ± 3* |
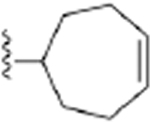
| 18 | 3,4-Cl2 |
|
241 ± 9* |
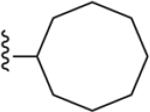
| 19 | 3,4-Cl2 |
|
146 ± 2* |
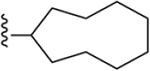
| 20 | 3,4-Cl2 |
|
241 ± 12* |
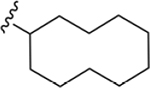
| 21 | 3,4-Cl2 |
|
135 ± 7* |
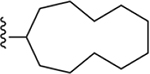
| 22 | 3,4-Cl2 |
|
122 ± 6* |
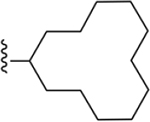
| 23 | 3,4-Cl2 |
|
101 ± 3 |
| 2-bicycloalkyl derivatives | |||
| 24 | 3,4-Cl2 |
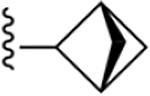
|
187 ± 14* |
| 25 | 3,4-Cl2 |
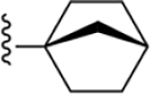
|
170 ± 6* |
| 26 | 3,4-Cl2 |
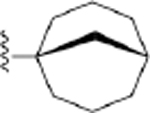
|
154 ± 7* |
| 27 | 3,4-Cl2 |
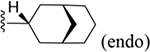
|
237 ± 12* |
| 28 | 3,4-Cl2 |
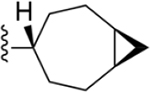
|
219 ± 16* |
| 29 | 3,4-Cl2 |
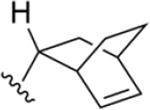
|
187 ±8* |
| 2-cycloalkyl derivatives with hydrophilic substitution | |||
| 30 | 3,4-Cl2 |
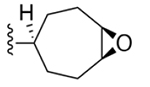
|
136 ± 11 |
| 31 | 3,4-Cl2 |
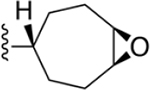
|
173 ± 14* |
| 32 | 3,4-Cl2 |
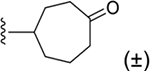
|
160 ± 16* |
| 33 | 3,4-Cl2 |
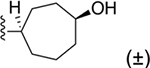
|
118 ± 14 |
| 34 | 3,4-Cl2 |
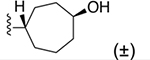
|
156 ± 10* |
| 2-alkyl and 2-cycloalkyl derivatives with modified 2-arylamino groups | |||
| 35 | 4-I |
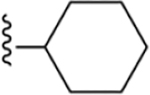
|
184 ± 9* |
| 36 | 4-Br |
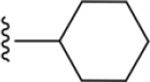
|
207 ± 17* |
| 37 |
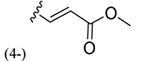
|
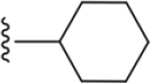
|
164 ± 10* |
| 38 |
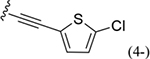
|
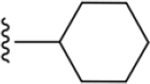
|
170 ± 12* |
| 39 | 4-I |
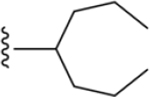
|
223 ± 10* |
| 48 | 4-Sn(CH3)3 |
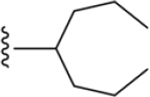
|
ND |
| 49 | 4-Sn((CH2)3CH3)3 |
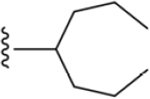
|
ND |
Effect of PAM derivative (1.0 μM) on [35S]GTPγS binding induced by 52 using WT hA3ARs (n = 3).
P ≤ 0.05 (one-way ANOVA with Bonferroni-adjusted t test for multiple comparisons) with respect to control in the absence of a PAM. Effects of 0.1 and 10 μM are shown in Table S4. ND, not determined.
