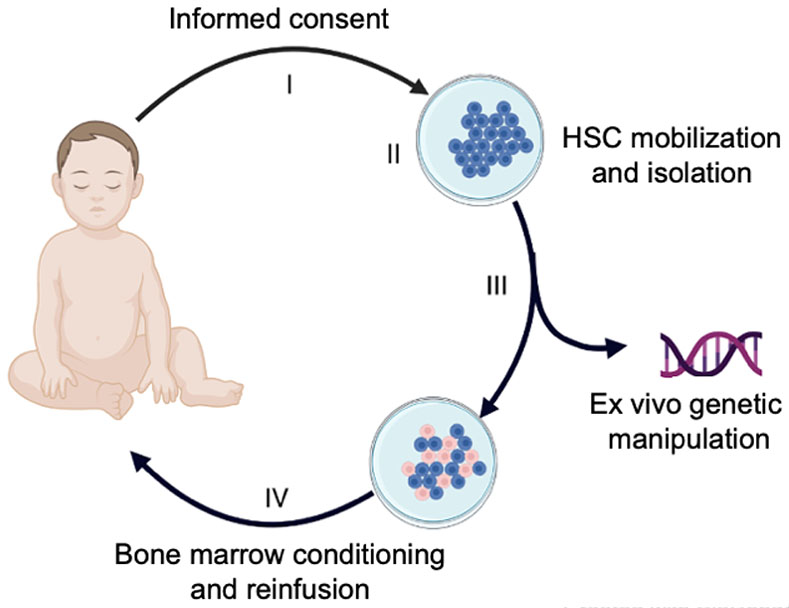
Figure 1. Gene therapy for transfusion-dependent β-thalassemia.
The multi-step process includes: (I) Informed consent, including patient/family education and written consent; (II) mobilization, apheresis collection and enrichment of patient (autologous) hematopoietic stem cells (HSCs); (III) Ex vivo genetic manipulation of HSCs to restore erythroid expression of β-globin or induce fetal hemoglobin (HbF) expression; (IV) Administration of bone marrow conditioning followed by infusion of the modified HSCs.