Abstract
The development of breast cancer (BC) is influenced by age, overweight, obesity, metabolic syndrome, and diabetes mellitus (DM), which are associated with hyperglycemia, glucose intolerance, insulin resistance, and oxidative stress. High glucose concentration increases a metastatic phenotype in cultured breast cancer cells, promoting cell proliferation, reactive species production (ROS), epithelial mesenchymal transition (EMT), and expression of proteolytic enzymes. Our aim was to determine whether diabetes mellitus favor BC progression in mice and its association with changes in the content of ROS and glycolytic and proteolytic enzymes. Diabetes was induced in 7-week-old Balb/c mice, under 6-h fasting with a unique i. p. dose of streptozotocin 120 mg/kg. Furthermore, 4T1 breast cancer cells were injected beneath the nipple to induce tumors. G6PD, GAPDH, ENO1, uPA, uPAR, PAI-1, β-catenin, Snail, vimentin, and E-cadherin were measured by western blot and MPP-9 and MMP-2 by gel zymography. TBARS were measured as markers of the lipid peroxidation. Lower survival and increased tumor growth, together with marked EMT, were found in diabetic in comparison with nondiabetic mice. The effects of diabetes were associated with enhanced lipid peroxidation and higher levels of glycolytic (G6PD, GAPDH, and ENO1) and proteolytic (uPA, MMP-9) enzymes. Possibly, hyperglycemia and ROS led to faster progression of breast cancer in diabetic mice, fomenting EMT and the expression of glycolytic and proteolytic enzymes. These enzymes participate in the supply of energy and precursors for macromolecular biosynthesis and extracellular matrix degradation during breast cancer progression.
Keywords: Diabetes, Breast cancer , uPA/uPAR system, Epithelial mesenchymal transition
Introduction
Type 2 diabetes mellitus (T2D) has been associated with increased risk for cancers of colon, breast, pancreas, liver, gall bladder, and non-Hodgkin lymphoma [1]. Therefore, diabetic patients have increased incidence of these types of cancer and worst survival [2, 3]. The link between T2D and breast cancer (BC) is supported by experimental and epidemiological studies. A variety of factors has been implied in the promotion of the growth and invasiveness of tumor cells by T2D, including hyperglycemia, insulin resistance, hyperinsulinemia, inflammatory processes, oxidative stress, ketone bodies production, and elevated levels of IGF-1 [4, 5]. High glucose concentration (HG) simulates the hyperglycemia as a diabetic condition, and the enhanced production of reactive oxygen species (ROS) has been recognized as an intermediary of the actions of both diabetes and HG [6–8]. ROS can damage DNA and induce genetic mutation or/and the expression of oncogenes. Through these mechanisms, elevated levels of ROS lead to cell proliferation, survival, migration, and invasiveness, contributing to tumorigenesis, tumor growth, and cancer progression [9].
The development of an aggressive cancer is associated with the epithelial mesenchymal transition (EMT); in this process, the cancer cells have reduced expressing cell adhesion proteins, including E-cadherin and claudin-1, and have increased expression of mesenchyme markers, such as vimentin, neural (N)–cadherin, Snail, and β-catenin; sometimes the latter two are dependent on the activation of the Wnt/β-catenin pathway [10, 11]. The EMT has been scarcely approached from the panorama of the development of cancer in a hyperglycemic microenvironment. It has been found that hyperglycemia facilitates metastasis by the induction of EMT and vascular destruction by oxidative stress. Hyperglycemia activates some signaling mechanisms that regulate the EMT, including the transforming growth factor β (TGF-β)/phosphatidylinositol 3-kinase (PI3K)/AKT pathway [8, 10].
Cancer cells undergo metabolic changes as one of the most important signs of the development and progression of cancer, including higher glucose uptake and glycolysis, favoring an increased rate of nucleotides (purines and pyrimidines), fatty acids, and amino acids biosynthesis; all of these processes promote the survival and proliferation of tumor cells [12]. Metabolism plays an important role in the establishment of tumors; Warburg suggested that cancer is, in reality, caused by a defect in the cellular energy metabolism through a process known as aerobic glycolysis [13]. Warburg observed that cancer cells consume more than the usual amount of glucose and generate ATP through the conversion of glucose into lactic acid, even in the presence of normal oxygen conditions [14, 15]. Hyperglycemia promotes glycolysis, inducing the expression of the glucose transporter GLUT1 and glycolytic enzymes, such as hexokinase, 6-phosphogluconate dehydrogenase, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), phosphoglycerate dehydrogenase, pyruvate kinase, lactate hydrogenase, pyruvate dehydrogenase, and enolase 1 (ENO1). These enzymes are induced by oncogenes that promote tumor development, including AKT, Ras, Myc, and HIF-1α [7, 12, 16].
The migratory and invasive activities of cancer cells imply the coordinated action of the plasminogen activator system and matrix metalloproteinases (MMP). The first begins the proteolytic cascade and involves the formation of plasmin by the urokinase-type plasminogen activator (uPA) which is enhanced by the binding of uPA to its surface receptor uPAR. Plasmin catalyzes the activation of some MMP and both degrade ECM proteins allowing tumor cell invasion and metastasis [17]. Increased levels of uPA and uPAR and PAI-1 have been associated with cancer progression [8, 17].
Considering that the effect of diabetes on breast cancer progression in vivo has been poorly analyzed, the objective of this study was to determine whether experimental diabetes mellitus promotes breast cancer progression in mice and if this effect is associated with a change of ROS and the EMT, and changes in the expression of some glycolytic and extracellular proteolytic enzymes.
Materials and Methods
Animals
Females Balb/c mice provided by the Animal Care Facility of FES Iztacala, UNAM, were used. The mice were maintained in a pathogen-free environment at our animal facility following the institutional and national guidelines. These experiments were approved by the FES-Iztacala Ethical Committee, UNAM. The mice were maintained at 22 °C, light-darkness cycles of 14 and 10 h, respectively, and food and water were provided ad libitum.
Induction of Experimental Diabetes in Balb/C Mice
Diabetes was induced in 7-week-old Balb/c mice, after a 6-h fast with a unique dose of streptozotocin (STZ) (Sigma-Aldrich, St. Louis, MO, USA) of 120 mg/kg intraperitoneally (i. p.), recently prepared, and dissolved in citrate buffer 0.05 M and pH 4.5 [18]. Glucose was measured using Accu-Check reactive slip (Roche Diagnostics, Indianapolis, IN, USA) in blood obtained through a small incision in the tail vein, and glycemia was recorded weekly for 7 weeks. The mice were randomly divided into two groups: (1) nondiabetics with breast cancer (BC) and diabetics with breast cancer (DM/BC). For DM/BC group, tumors were induced in mice with glycemia between 200 and 250 mg/dl after 1 week of STZ administration, and only animals which glycemia increased further were considered diabetics and remained in this study. Twenty-four mice were employed for the evaluation of survival and followed for 8 weeks after STZ administration. Considering the low survival of DM/BC group at 8 weeks, for the other evaluations, mice were studied at 7 weeks after STZ administration, and an additional group of 48 mice was distributed equally between BC and DM/BC groups.
Cell Culture and Induction of Tumors
The 4T1 breast cancer cell line purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) was grown in Dulbecco’s Modified Eagle’s Medium (Sigma-Aldrich Co., St. Louis, MO, USA) with 10% fetal bovine serum (Biowest, Nuaille, France) containing penicillin (100 U/ml) and streptomycin (100 μg/ml (Life Technologies, Inc. BLR, Grand Island, NY, USA). The cells were grown in 75-cm2 tissue culture flasks in a humidified 5% CO2, 95% air atmosphere in a CO2 incubator at 37 °C. Normoglycemic and hyperglycemic mice were injected subcutaneously under the fourth nipple of the mammary gland, with 1 × 103 4T1cells in 50 μl of isotonic solution. The area of injection was previously shaved and disinfected [19]. Mice were sacrificed 7 weeks (49 days) later of the induction of tumors. Tumors were weighed and groups of 2 or 3 tumors were pooled and processed separately. Survival was recorded for 8 weeks following tumor induction.
Evaluation of Thiobarbituric Acid Reactive Species (TBARS)
Since it has been considered that ROS and oxidative stress are two of the major mediators of the actions of diabetes [6], promote the progression of some cancers and tumor growth [8–10], and also mediate the proliferative effect of high glucose on breast cancer cells [6], TBARS were evaluated as an indicator of oxidative stress and an indirect estimation of ROS concentration. TBARS evaluate lipid peroxidation products, principally malondialdehyde (MDA), and are dependent on the concentration of ROS. MDA concentration was measured according to the method of thiobarbituric acid (TBA) [20]. The tumors were obtained, cleaned carefully, fragmented, and homogenized in 1.15% (w/v) cold KCl solution at a ratio of 1/10. The homogenate was centrifuged at 3000 g for 15 min, and during the procedure, 0.5 ml of 20% trichloroacetic acid was added to 0.5 ml of the tissue homogenate, and further 1 ml of 0.67% TBA was added. The mixture was incubated at 90–100 °C for 15 min and cooled, and then 4 ml of n-butanol was added and centrifuged at 3000 rpm for 15 min. The supernatant was separated for the measurement of absorbance at 535 nm. Lipid peroxidation products were expressed as the MDA content in nanomoles per mg of proteins [20, 21].
Western Blot Analysis
The tumors were homogenized in 100 μl of RIPA-Tris lysis buffer (Tris 50 mM pH 7.4, NaCl 150 mM, Triton X-100 1%, sodium deoxycholate 1%, and SDS 0.1%) containing protease inhibitors (EGTA 2 mM, PMSF 100 mM, aprotinin 2 μg/ml, leupeptin 2 μg/ml, and EDTA 100 mM) and were maintained under constant shaking for 3 h at 4 °C. Subsequently, the samples were centrifuged for 5 min at 20,800 rpm, and the supernatant (80 μg of protein) was denatured in Laemmli sample buffer [22], resolved through 10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (SDS-PAGE), and electroblotted onto polyvinylidene difluoride (PVDF) membranes. Blots were stained with Ponceau S to confirm that protein loading was identical in all lanes. Membranes were soaked in PBS to remove the Ponceau S and incubated for 90 min in Tris-buffered saline (TBS) containing 5% dried skimmed milk and 0.1% Tween 20 to block non-specific protein binding sites. Subsequently, the membranes were incubated for 24 h at 4 °C with the primary antibodies 1:500 G6PD, GADPH, ENO-1, PAI-1, β-actin, uPA, uPAR, and enolase from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Additionally for the evaluation of EMT, β-catenin, Snail, and TCF8/ZEB1 antibodies of Cell Signaling (Cambridge, MA, USA) were used after their dilution in TBS-Tween 20 0.1% including 5% dried skimmed milk. Then, membranes were washed and incubated with peroxidase-conjugated secondary antibodies 1:10,000. Antibody binding was detected using the Clarity ECL western blot detection kit (Bio-Rad, Hercules, CA, USA), following the supplier’s instructions. Antibodies are described in Table 1. The blots were subjected to densitometry evaluation and data were analyzed using GraphPad Prism ver. 5 software (San Diego, CA, USA). The intensity of the bands was calculated after background subtraction and normalization to β-actin. Western blots were repeated three times. Prestained molecular weight markers 27–180 (SDS-7B, Sigma-Aldrich Co., St. Louis, MO, USA) were run on the same gels for molecular weight estimation. Proteins were quantitated by the modified Lowry method [23] in the samples for this technique and for gel zymography.
Table 1.
Antibodies for western blot
| Antibody | Dilution | Catalog number | Source |
|---|---|---|---|
| Anti-vimentin | 1:500 | 5741 | Cell Signaling Technology |
| Anti-E-cadherin | 1:500 | 3195 | Cell Signaling Technology |
| Anti-β-catenin | 1:500 | 8480 | Cell Signaling Technology |
| Anti-Snail | 1:500 | 3879 | Cell Signaling Technology |
| Anti-TCF8 | 1:500 | 3396 | Cell Signaling Technology |
| Anti-GAPDH | 1:500 | Sc-17840 | Santa Cruz Biotechnology |
| Anti-G6PD | 1:500 | Sc-373886 | Santa Cruz Biotechnology |
| Anti-enolase 1 | 1:500 | Sc-365155 | Santa Cruz Biotechnology |
| Anti-uPA | 1:500 | Sc-59729 | Santa Cruz Biotechnology |
| Anti-uPAR | 1:500 | Sc-376494 | Santa Cruz Biotechnology |
| Anti-PAI-1 | 1:500 | Sc-5297 | Santa Cruz Biotechnology |
| Anti-β-actin | 1:500 | Sc-8432 | Santa Cruz Biotechnology |
Gel Zymography
The tumors were homogenized in cold lysis buffer (25 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 1% Nonidet P-40) and centrifuged for 5 min at 1300 g, and levels a zymogen and active forms of MMP-2 and MMP-9 were analyzed by gelatin zymography [24, 25]. The supernatant containing 60 μg of proteins was separated by 10% SDS-PAGE, using gels copolymerized with 0.15% w/v of porcine gelatin (G2625, Sigma-Aldrich Co., St. Louis, MO, USA) in nonreducing condition. After electrophoresis, gels were washed twice with 2.5% Triton X-100 for 30 min at room temperature to remove SDS. The gels were then developed at 37 °C for 48 h using 50 mM Tris–HCl, pH 7.5, containing 200 mM NaCl and 10 mM CaCl2. Gels were stained with Coomassie Brilliant Blue R (0.1% w/v) and destained in 30% methanol with 10% acetic acid.
The plasminogen activators were examined by casein-plasminogen zymography. The proteins (80 μg) of tumor homogenates were separated by 10% SDS-PAGE, using gels copolymerized with 1 mg/ml of β-casein and 10 μg/ml of human plasminogen. After electrophoresis, gels were washed twice with 2.5% Triton X-100 for 30 min and then incubated at 37 °C for 24 h in 100 mM glycine, 10 mM EDTA, and pH 8.3. Gels were stained and destained as described before. Gels exclusively copolymerized with casein were used to exclude plasminogen-independent caseinolytic bands.
Proteases were detected as clear zones of degradation against a blue background. The zymograms were imaged using a G:Box Gene Documentation System (Syngene, Frederick, MD, USA), and zones of enzyme activity were quantified using Image J software (NIH). Densitometry data of gelatinases were expressed as folds of change, with the BC group set to 1.
Besides, TBARS and gelatinases were evaluated in the liver of diabetic mice without diabetes to corroborate that some effects of diabetes are not exclusives of tumors; in this case, diabetes was induced in CD1 mice using STZ and sacrificed after 2 weeks of STZ administration. Livers were processed following the procedure used for the tumors.
Statistical Analysis
GraphPad Prism statistical software 5 was used to analyze the data. Independent sample Student’s t test was employed for the comparisons between DM/BC and BC groups. Survival curves were plotted using the Kaplan-Meier method, and the differences among both groups were compared using the log-rank (Mantel-Cox) test.
Results
Diabetes Worsened Survival and Promoted Loss of Corporal Weight
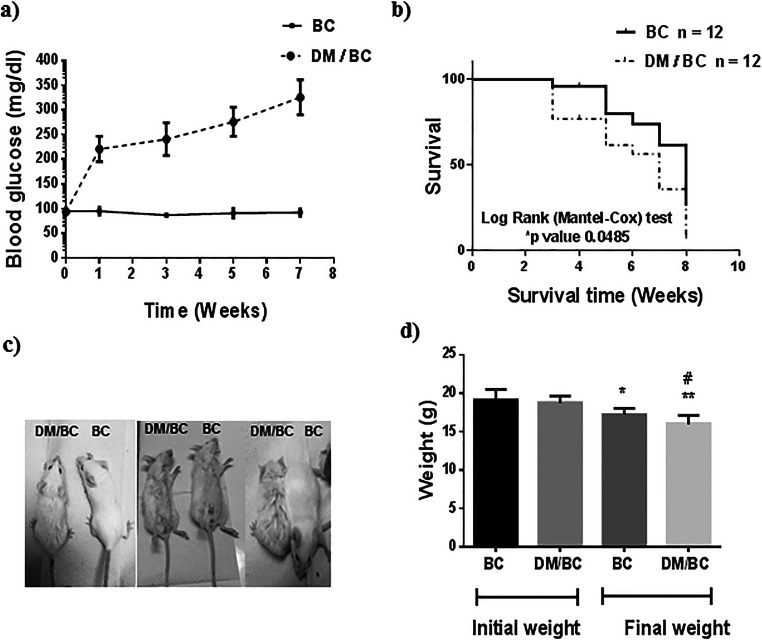
Blood glucose concentrations were followed as an indication of the diabetic condition of mice (Fig. 1a). At the first week post STZ administration, the blood glucose was between 200 and 250 mg/dl, and further glycemia progressively increased until reaching values from 280 to 350 mg/dl at week 7 after diabetes induction. When STZ was not administered (BC group), blood glucose remained in the range of 80–100 mg/dl, without significant changes during the tumor development. On the other hand, mice with breast cancer lost weight in the presence or absence of diabetes. However, the weight loss was higher in the diabetic mice than in nondiabetic (Fig. 1c and d).
Fig. 1.
Diabetes decreased survival and corporal weight of mice with breast cancer. Diabetes was induced by STZ in 7-week mice and breast tumors 1 week after in normoglycemic (BC) and diabetic (DM/BC) mice. Glycemia (a) and survival (b) were followed after STZ administration in 12 mice per condition in separated experiments, glycemia by 7 weeks and survival by 8 weeks after STZ administration. Overall survival was evaluated by Kaplan-Meier curves and log-rank (Mantel-Cox) test; p indicated that DM/BC had lower survival than BC. c Representative images of mice with breast cancer, after 7 weeks of STZ administration are presented. d Mice weight, n of 24 mice, were used per group for the initial weight, and the values of n were of 15 and 8 for final weights of BC and DM/BC, respectively. Means ± SD, *p ≤ 0.05 and **p ≤ 0.01 versus its respective initial weight, #p ≤ 0.05 versus BC final weight
The Kaplan-Meier plots of survival of BC and DM/BC mice are presented in Fig. 1b; a higher overall mortality rate was observed in cancerous diabetic mice (DM/BC) than in nondiabetics (p = 0.0406). At 3 weeks after tumor induction, the DM/BC group exhibited a survival of 76.3%, while in the BC group, it was 95%. Whereas at 7 weeks of the follow-up, 61.43% of BC mice survive, while only 35% of DM/BC mice survive.
The diabetic state after STZ administration was consistent with the progressively increased levels of glycemia, and this state was associated with poor survival (bad prognostic) and high weight loss in mice with BC.
Diabetes Mellitus Promoted Tumor Growth and Induced Lipid Peroxidation
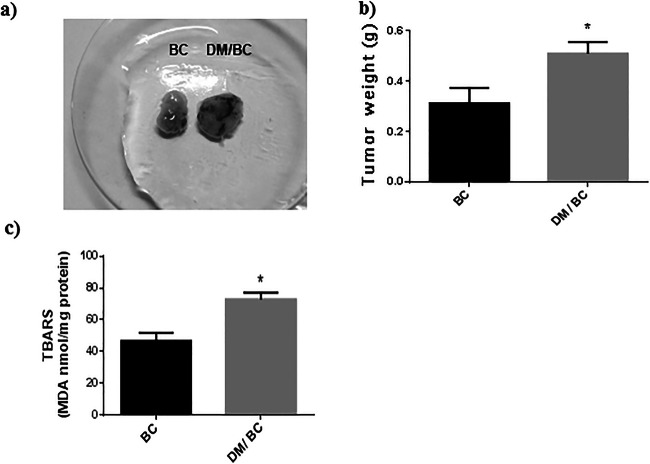
On the other hand, the tumors of diabetic mice reached higher weights than those of nondiabetics (Fig. 2a and b), indicating that diabetes promoted tumor growth in comparison with the nondiabetic mice.
Fig. 2.
Diabetes increased the growth and lipid peroxidation products of breast tumors. Breast tumors (a), tumor weight (b), and TBARS (c) were obtained at 6 weeks of cancer induction in nondiabetic (BC) or diabetic (DM/BC) mice. Representative images and weights of 15 (BC) or 8 (DM/BC) tumors are presented. TBARS were measured in three independent samples; each one obtained from 2 or 3 tumors and realized by triplicate. Means ± SD, *p < 0.05 versus BC
Because ROS can mediate some effects of diabetes and activate oncogenes and promote tumor growth [6, 26], lipid peroxidation products were evaluated using the technique of TBARS as one of the indicators of oxidative damage of lipids and therefore changes in the levels of ROS. In this study a 45% higher TBARS concentration in the tumors of the DM/BC group was found, in relation to those of the BC group (Fig. 2c). These results indicate that the tumors developed in diabetic mice had increased ROS levels and oxidative stress than those of nondiabetic condition and that the effects of diabetes can be mediated by ROS.
Diabetes Mellitus Enhanced the Epithelial Mesenchymal Transition
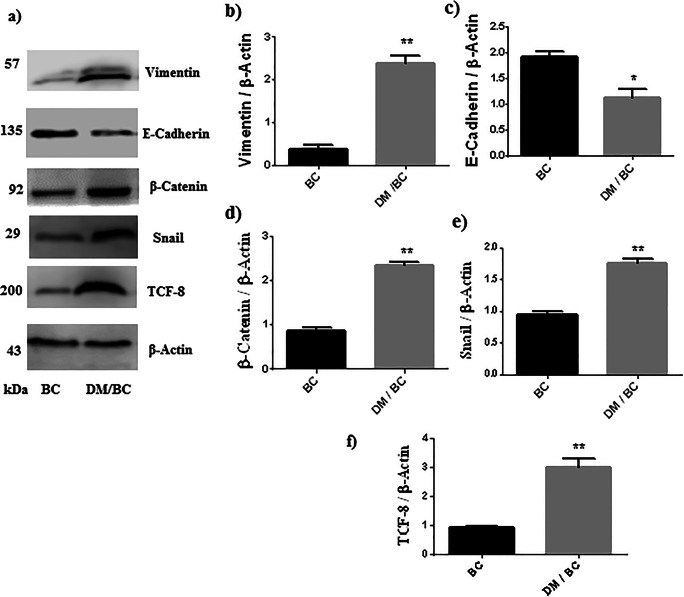
The previous data indicated that diabetes promoted breast cancer, which has been associated with a mesenchymal and migratory phenotype originated by the EMT; therefore, a variety of proteins that participate (vimentin, E-cadherin) or regulate (TCF8, Snail, β-catenin) this process were evaluated. The expression of vimentin was higher in the DM/BC group in comparison with the control group (Fig. 3a and b), while the level of E-cadherin was diminished in the DM/BC group (Fig. 3a and c). These changes indicate the loss of epithelial characteristics (strong adhesiveness between cells) and the acquisition of a mesenchymal phenotype. The levels of positive regulators of EMT (TCF-8, Snail, and β-catenin) were higher in the DM/BC group than those of the BC group (Fig. 3a, d, e, and f), suggesting that EMT in breast tumors was promoted by diabetes.
Fig. 3.
Diabetes promoted EMT. Vimentin, E-cadherin, β-catenin, Snail, and TCF-8 in tumor lysate were analyzed by western blot (a) and densitometry analysis (b, c, d, e, and f, respectively); the intensity of the band was calculated after background subtraction and normalization to β-actin. Representative images of zymograms and means ± SD of three independent samples, each one obtained from 2 or 3 tumors, are presented,*p < 0.05 or **p < 0.01 versus BC
Diabetes Was Associated with High Expression of Glycolytic Enzymes
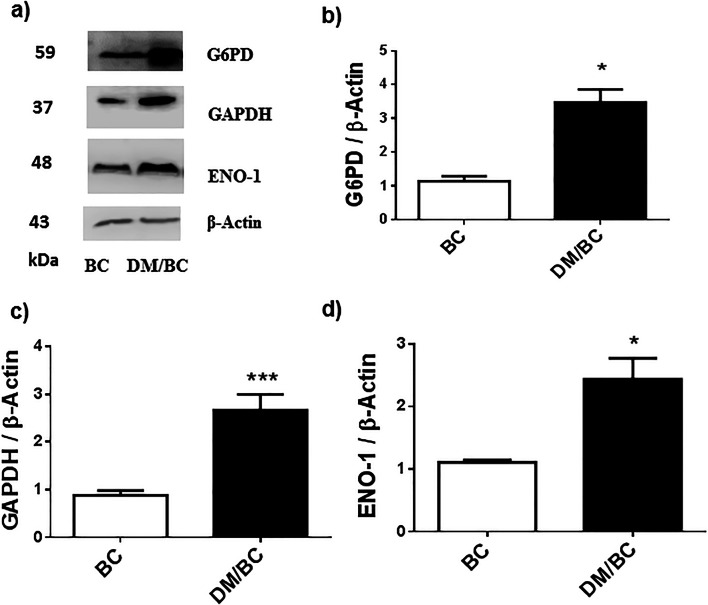
The concentrations of glucose metabolism enzymes in tumors of diabetic mice were found to be highly modified (Fig. 4); these tumors presented statistically significant higher expression of enzymes of the pentose phosphate pathway or PPP (glucose 6 phosphate dehydrogenase or G6PD) and glycolysis (glyceraldehyde 3 phosphate dehydrogenase or GAPDH and enolase 1 or ENO 1) in comparison with tumors of normoglycemic mice (Fig. 4a–d). For the G6PD, GAPDH, and ENO 1, respective increases of 89%, 400%, and 87% were found in the DM/BC group (p ≤ 0.05). These data reveal that diabetes induced changes in the concentration of glucose metabolic enzymes that can indicate increased PPP and glycolysis pathways, which can be associated with metabolic dysregulation and progression of breast cancer.
Fig. 4.
Diabetes promoted the expression of glucose metabolism enzymes in breast cancer. The tumors of BC and DM/BC groups were analyzed by western blot (a). The intensity of the bands of G6PD, GADPH, and ENO1 was quantified by densitometry analysis (b, c, and d, respectively), and the intensity of the bands was calculated after background subtraction and normalization to β-actin. Representative images of blots and means ± SD of three independent samples, each one obtained from 2 or 3 tumors, are presented, *p ≤ 0.05 or ***p ≤ 0.001 versus BC
Diabetes Increased the Expression of Components of the Plasminogen Activator System
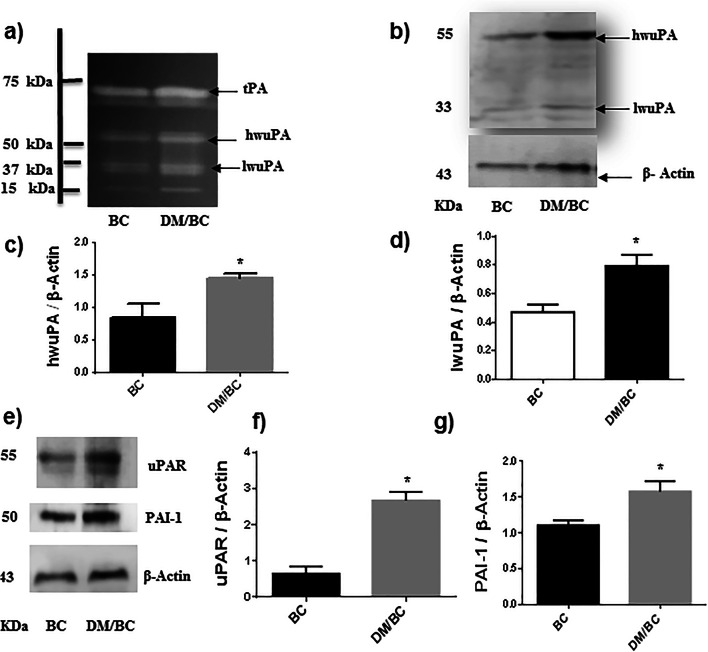
Because the above data indicated that diabetes promoted the progression of breast cancer, some components of extracellular proteolytic systems associated with the migratory and invasive ability of cells were evaluated. First, plasminogen-casein zymography for plasminogen activators shows several proteolytic bands of molecular weights of 67, 55, 33, and 15 kDa (Fig. 5a). The first band probably corresponds with the tissue-type plasminogen activator (tPA), the band of 55 kDa with high molecular weight uPA (hwuPA), and the band of 33 kDa with low molecular weight uPA (lwuPA). The identity of the uPA bands of 55 and 33 kDa was confirmed by western blot (Fig. 5b). The uPA is secreted as single chain inactive uPA (Pro-uPA) of 50–55 kDa, Pro-uPA is transformed to active two chains uPA of the same molecular weight, and furthermore the low molecular weight uPA is produced by the loss of some of its non-catalytic domains. The hwuPA corresponds predominantly with the active two chains uPA.
Fig. 5.
Diabetes increased the expression of components of the plasminogen activation system. The uPA was assessed by zymography in polyacrylamide gels copolymerized with casein-plasminogen (a). Additionally, uPA (b, c, and d), uPAR (e and f), and PAI-1 (e and g) were analyzed by western blot and densitometry analysis; the intensity of the bands was calculated after background subtraction and normalization to β-actin. Representative images of blots and means ± SD of three independent samples, each one obtained from 2 or 3 tumors, are presented, *p < 0.05 versus BC
In the zymogram, tumors of the DM/BC group exhibited qualitatively higher enzymatic activity of the hwuPA and lwuPA, in comparison with those of the BC groups (Fig. 5a). Similar results for uPA content were obtained by western blot (Fig. 5b). In this case, the intensity of hwuPA and lwuPA in the DM/BC group were 75% and 68%, respectively higher than those of the BC group (p ≤ 0.05; Fig. 5c and d). The expression of uPAR in the DM/BC group reveals a significant increase of 250% in comparison with that of the BC group (p ≤ 0.05; Fig. 5e and f). The concentration of PAI-1 was 57% higher in the DM/BC group than that of the BC group (p ≤ 0.05; Fig. 5e and g). These changes suggest that diabetes promoted a more migratory and invasive phenotype in breast cancer cells.
Diabetes Mellitus Favored the Expression of Gelatinases
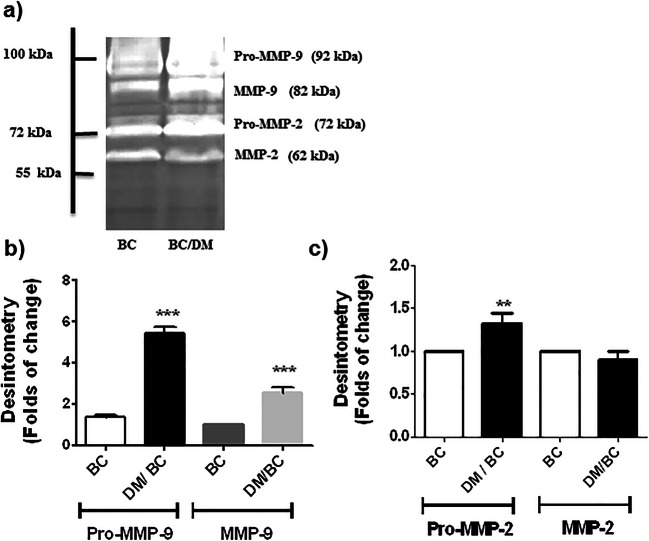
Gelatinases (MMP-9 and MMP-2) were evaluated because they are constituents of degradative proteolytic cascade driven by uPA involved in the degradation of the ECM. Gelatin zymography revealed three major EDTA-sensitive bands of 92, 82, and 72 kDa presented in the breast tumors. The first was the most strongly detected and corresponds with the proenzyme of MMP-9 (Pro-MMP9), while the band of 82 kDa is the active MMP-9, and the bands of 72 kDa and 62 kDa correspond with Pro-MMP-2 and active MMP-2, respectively (Fig. 6a). This procedure evaluates the presence and levels of precursors and active forms of MMP-9 and 2. Pro-MMP-9 and MMP-9 levels were 2.53 times higher in the tumors of the DM/BC group in comparison with those of the BC group (p ≤ 0.001; Fig. 6a and b). Pro-MMP-2 level was 1.4 times higher in the experimental DM/BC group, in comparison with the BC group (p ≤ 0.01). However, no significant changes were found for MMP-2. These data indicate that diabetes induced higher levels of gelatinases associated with the remodeling of the ECM and favoring a migratory and invasive phenotype of breast cancer.
Fig. 6.
The precursors and active forms of MMP-9 and MMP-2 are increased by diabetes. The gelatinases (MMP-9 and MMP-2) were assessed by zymography in acrylamide gels copolymerized with gelatin (a), followed by densitometry analysis (b, c). Data in b and c were expressed as folds of change, with the BC group set to 1. Representative images and means ± SD of three independent samples, each one obtained from 2 or 3 tumors, are presented, **p ≤ 0.01; ***p < 0.001 versus BC
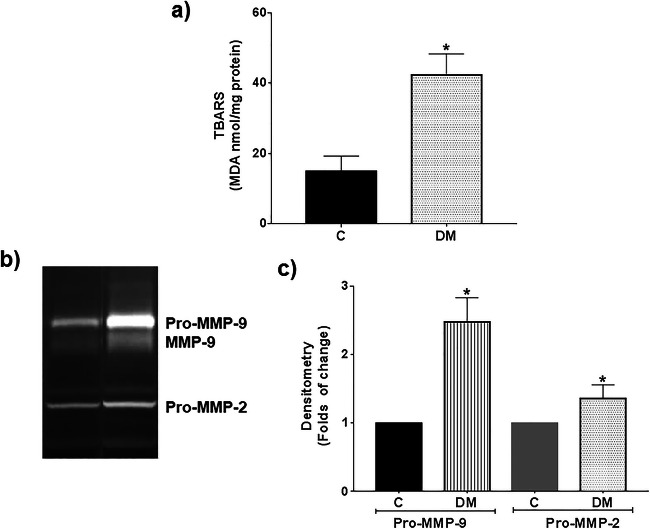
Diabetes Increased TBARS and Gelatinases in the Liver of Mice Without Breast Cancer
Higher values of 176%, 159%, and 40% for TBARS, Pro-MMP-9, and Pro-MMP-2, respectively, were found in livers of diabetic mice compared with nondiabetics (Fig. 7). These results corroborate that diabetes affected other organs and, similar to its effects in tumors, induce higher levels of ROS and MMP-9 and MMP-2, including the presence of the active form of MMP-9 (Fig. 7b).
Fig. 7.
Diabetes increased TBARS and gelatinases in the liver of mice without cancer. Diabetes was induced in CD1 mice at 7 weeks of age using STZ. TBARS (a) and gelatinases (b and c) were evaluated in the liver of nondiabetic (C) and diabetic (DM) mice. Representative images and means ± SD of three independent livers are presented, *p ≤ 0.05 versus C
Discussion
Diabetes and cancer are two major health problems worldwide. The association between these diseases denotes poor prognosis, because through a variety of conditions diabetes promotes the initiation, progression, and metastasis of breast cancer, including hyperglycemia and oxidative stress. According to the tumor-promoting action of diabetes, in this study, it was found that diabetes induced the rapid growth and a migratory and invasive phenotype in breast tumors. Diabetic mice were more susceptible to damage induced by breast cancer, presenting diminished survival and higher weight loss. The promotion of cancer progression by diabetes was associated with enhanced products of lipid peroxidation, together with increased concentration of diverse proteins implicated in EMT (vimentin, E-cadherin, TCF-8, Snail, and β-catenin), glycolysis (GAPDH and ENO-1), pentose phosphate pathway (G6PD), and tissue remodeling (uPA, uPAR, PAI-1, MMP-9, and MMP-2). All of these changes are characteristics of a more aggressive cancer and confirmed that diabetes promoted cancer progression [1–3, 27].
Diabetes is a complex disease and multiple factors can mediate its action on cancer. Elevated glycemia and higher TBARS in tumors observed in diabetic mice suggest that hyperglycemia and ROS must be, at least partially, some of the factors involved in promoting the progression of breast cancer by diabetes. The concentration of TBARS is proportional to ROS levels and is considered an indicator of oxidative stress. Prior studies have found that high glucose and diabetes induce the production of ROS in breast cancer cell lines [8, 28] or in different tissues of diabetic animals [6]. Additionally, the proliferative, pro-migratory, and pro-invasive abilities of pancreatic and breast cancer cells induced by high-glucose are mediated by ROS [8, 29]. In fact, other factors can mediate the effects of diabetes, but they were not evaluated here, for example, ketone bodies. Diabetes is highly ketogenic and ketone bodies are elevated in diabetic patients. In addition, studies on breast cancer have shown that ketone bodies and their utilization drive tumor growth and metastasis [5, 30]. It could be interesting to evaluate the participation of ketone bodies as mediators of the action of diabetes on cancer progression, since this possibility has been only suggested [5]. Additionally, diabetes affects other tissues and organs and systemic conditions, which can induce changes in tumors too. Diabetes had similar effects on some tissues, like those of liver and tumors; however, their properties are altered according to their characteristics.
The acquisition of a mesenchymal phenotype is associated with the aggressiveness of tumor cells [31]. The findings that diabetes increased the expression of vimentin (a mesenchymal marker) and reduced the levels of E-cadherin (an epithelial marker) in breast tumors suggested the enhanced mesenchymal characteristic and the loss of epithelial phenotype. These changes imply the loss of stable links between cells and the acquisition of cells with higher migratory ability, facilitating the spreading of tumor cells. Elevated expression of vimentin has been associated with poor prognosis and cancer progression [32, 33].
The effect of diabetes on the EMT was also supported by the increased concentration of transcription factors (β-catenin, Snail, and TCF8/ZEB1) involved in the induction of EMT. These transcription factors are regulated by hyperglycemia and some glycolytic enzymes. Suppression of ENO-1 partially prevented the induction of EMT by high glucose on gastric cancer cells, and it has been suggested that EMT was triggered by this enzyme [34]. In line with this suggestion, Snail and ENO-1 were found to increase in the tumors of diabetic mice, suggesting that both proteins were implicated in the action of diabetes in cancer progression. Moreover GAPDH, another glycolytic enzyme that was also increased in breast tumors by diabetes, promoted the expression of Snail, when this enzyme is translocated into the nucleus, through its interaction with the transcriptional factor Sp1, leading to EMT [35].
Our data indicate that diabetes favored the glycolysis in breast tumors, because the concentrations of GAPDH and ENO1 were increased. Elevated glycolysis in cancer cells provides an adequate supply of energy and intermediary metabolites required for the formation of precursors for the synthesis of macromolecules and to support their rapid growth and proliferation [13, 28, 36]. The induction of several enzymes of glycolysis support increased glycolysis and has been associated with breast cancer promotion [37]. GAPDH can be utilized to estimate metabolic activity, tumor aggressiveness, and response to chemotherapy in cancer [38]. The inhibition of GADPH by means of siRNA in colon cancer cells decreased the glycolytic flux and inhibited the migration and invasion [39].
The canalization of metabolites for biosynthesis was also supported by the increased concentration of G6PD in tumors of diabetic mice; this enzyme is the major resource of NADPH + H+, coenzyme required for the biosynthesis of macromolecules and for the antioxidant defense. Additionally, G6PD is the rate-limiting enzyme of the PPP, the principal resource of ribose for the synthesis of nucleotides and nucleic acids. The relevance of G6PD in cancer is supported by several evidences. Elevated concentration of G6PD is an indicator of the negative prognosis of breast cancer [40]. Increased G6PDH expression drives the PPP flux, supporting biomass synthesis for cancer cell proliferation and tumor progression and also was associated with resistance to tamoxifen [39, 40]. Suppression or inhibition of G6PD increased the glycolytic flux and glutamine uptake and reduced lipid synthesis leading to the inhibition of proliferation and survival of breast cancer cells [39]. It has been found that the expression of G6PD in MCF-7 and MDA-MB-231 cell lines was upregulated by the nuclear factor, erythroid 2-like 2 (Nrf2), promoting their proliferative, migratory, and invasive abilities [41].
On the other hand, the increased expression of components of proteolytic systems involved in tissue remodeling and invasion, uPA, uPAR, PAI-1, and MMP-9 in tumors of diabetic animals indicated that diabetes promoted the migratory and invasive activity of tumor cells. Plasminogen is activated by uPA forming plasmin, and this enzyme can degrade various components of the ECM, such as fibrin and collagen, and participate in the activation of the metalloproteinases like MMP-9 and MMP-2 [42]. Previously, we found that the high glucose induction of an invasive phenotype in MDA-MB-231 was associated with higher expression of uPA, uPAR, and PAI-1 and was prevented by an inhibitor of plasminogen activation [43].
In the other hand, the participation of Wnt in the promotion of an aggressive phenotype by diabetes was suggested because the levels of β-catenin and TCF8 was increased in BC tumors of diabetic mice, and Wnt promotes EMT and progression of human breast cancer through the activation of β-catenin/TCF8 complex [11]. Additionally, this finding is consistent with the association between increased expression of TCF8/ZEB1 with the invasiveness of colon, lung, and breast cancer cell lines [44, 45]. The increase of TCF8/ZEB1 has been found to inhibit the expression of factors that participate in cellular polarity and the synthesis of the basal membrane, together with the increase of the expression of MMP-1, MMP-9, and MMP-14, thus promoting the remodeling of the basal membrane and the invasive process.
Conclusion
The increased concentration of some enzymes of glucose metabolism (G6PD, GAPDH, and ENO1), proteins of the EMT (vimentin, Snail, β-catenin and TCF8), and proteolytic systems (uPA, uPAR, PAI-1,and MMP-9) in BC tumors of diabetic mice indicated that diabetes promoted glycolysis and expression of GADPH and ENO1, which in collaboration with Wnt/β-catenin/TCF8 signaling induced the EMT and the ECM remodeling by plasmin and matrix metalloproteinases, favoring the progression of breast cancer. Considering the studies of the action of high glucose in cancer cell cultures and the increase of TBARS observed in this study, the action of diabetes is mediated, at least partially, by hyperglycemia and ROS. These mechanisms could favor the colonization of aggressive cancers associated with resistance to chemotherapy, negative prognosis, and poor survival of diabetic patients with breast cancer.
Funding Information
This work was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Dirección General de Asuntos del Personal Académico (DGAPA), and Universidad Nacional Autónoma de México (UNAM), projects IN220818 and IA206920. Viedma-Rodríguez R was postdoctoral fellow of DGAPA, UNAM, during the realization of this study.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collins KK. The diabetes-cancer link. Diabetes Spectr. 2014;27(4):276–280. doi: 10.2337/diaspect.27.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer. 2012;19(6):793–803. doi: 10.1530/ERC-12-0242. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Zhang X, Sang H, Zhou Y, Shang C, Wang Y, Zhu H. Effects of hyperglycemia on the progression of tumor diseases. J Exp Clin Cancer Res. 2019;38(1):327. doi: 10.1186/s13046-019-1309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis. Cell Cycle. 2010;9(17):3506–3514. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Díaz-Flores M, Baiza-Gutman LA. The diabetes textbook: Clinical principles, patient management and public health issues. Switzerland: Springer Nature; 2019. Biochemical mechanisms of vascular complications in diabetes; pp. 695–707. [Google Scholar]
- 7.Ramteke P, Deb A, Shepal V, Bhat MK. Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality. Cancers. 2019;11(9):1402. doi: 10.3390/cancers11091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores-López LA, Martínez-Hernández MG, Viedma-Rodríguez R, Díaz-Flores M, Baiza-Gutman LA. High glucose and insulin enhance uPA expression, ROS formation and invasiveness in breast cancer-derived cells. Cell Oncol. 2016;39(4):365–378. doi: 10.1007/s13402-016-0282-8. [DOI] [PubMed] [Google Scholar]
- 9.Klaunig JE, Wang Z. Oxidative stress in carcinogenesis. Curr Opin Toxicol. 2018;7:116–121. doi: 10.1016/j.cotox.2017.11.014. [DOI] [Google Scholar]
- 10.Wu J, Chen J, Xi Y, Wang F, Sha H, Luo L, Zhu Y, Hong X, Bu S. High glucose induces epithelial-mesenchymal transition and results in the migration and invasion of colorectal cancer cells. Exp Ther Med. 2018;16(1):222–230. doi: 10.3892/etm.2018.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yook JI, Li X-Y, Ota I, Hu C, Kim HS, Kim NH, Cha SY, Ryu JK, Choi YJ, Kim J, Fearon ER, Weiss SJ. A Wnt–Axin2–GSK3β cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8(12):1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 12.Fadaka A, Ajiboye B, Ojo O, Adewale O, Olayide I, Emuowhochere R. Biology of glucose metabolization in cancer cells. J Oncol Sci. 2017;3(2):45–51. doi: 10.1016/j.jons.2017.06.002. [DOI] [Google Scholar]
- 13.Schwartz L, Seyfried T, Alfarouk KO, Da Veiga MJ, Fais S. Out of Warburg effect: an effective cancer treatment targeting the tumor specific metabolism and dysregulated pH. Semin Cancer Biol. 2017;43:134–138. doi: 10.1016/j.semcancer.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Vasconcelos-dos-Santos A, de Queiroz RM, da Costa RB, Todeschini AR, Dias WB. Hyperglycemia and aberrant O-GlcNAcylation: contributions to tumor progression. J Bioenerg Biomembr. 2018;50(3):175–187. doi: 10.1007/s10863-017-9740-x. [DOI] [PubMed] [Google Scholar]
- 15.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phan LM, Yeung S-CJ, Lee M-H. Cancer metabolic reprogramming: importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol Med. 2014;11(1):1–19. doi: 10.7497/j.issn.2095-3941.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mekkawy AH, Pourgholami MH, Morris DL. Involvement of urokinase-type plasminogen activator system in cancer: an overview. Med Res Rev. 2014;34(5):918–956. doi: 10.1002/med.21308. [DOI] [PubMed] [Google Scholar]
- 18.Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A, Eberhardt NL, Kudva YC. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. doi: 10.1258/la.2010.010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2000;39(1):20.22.21–20.22.16. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 20.Sheu JY, Chen PH, Tseng WC, Chen CY, Tsai LY, Huang YL. Spectrophotometric determination of a thiobarbituric acid-reactive substance in human hair. Anal Sci. 2003;19(6):957–960. doi: 10.2116/analsci.19.957. [DOI] [PubMed] [Google Scholar]
- 21.Gonenc A, Ozkan Y, Torun M, Simsek B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J Clin Pharm Ther. 2001;26(2):141–144. doi: 10.1046/j.1365-2710.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Lowry OH, Rosebrough NJ, Farr A, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275. doi: 10.1016/S0021-9258(19)52451-6. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Hernandez MG, Baiza-Gutman LA, Castillo-Trapala A, Armant DR. Regulation of proteinases during mouse peri-implantation development: urokinase-type plasminogen activator expression and cross talk with matrix metalloproteinase 9. Reproduction. 2011;141(2):227–239. doi: 10.1530/REP-10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth M, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9 by gelatin zymography. Methods Mol Med 2001;57:163–174 [DOI] [PMC free article] [PubMed]
- 26.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krukovets I, Legerski M, Sul P, Stenina-Adognravi O. Inhibition of hyperglycemia-induced angiogenesis and breast cancer tumor growth by systemic injection of microRNA-467 antagonist. FASEB J. 2015;29(9):3726–3736. doi: 10.1096/fj.14-267799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshmukh A, Arfuso F, Newsholme P, Dharmarajan A (2018) Regulation of cancer stem cell metabolism by secreted frizzled-related protein 4 (sFRP4). Cancers:10(2) [DOI] [PMC free article] [PubMed]
- 29.Li W, Zhang L, Chen X, Jiang Z, Zong L, Ma Q. Hyperglycemia promotes the epithelial-mesenchymal transition of pancreatic cancer via hydrogen peroxide. Oxidative Med Cell Longev. 2016;2016:5190314. doi: 10.1155/2016/5190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11(21):3964–3971. doi: 10.4161/cc.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phoomak C, Vaeteewoottacharn K, Silsirivanit A, Saengboonmee C, Seubwai W, Sawanyawisuth K, Wongkham C, Wongkham S. High glucose levels boost the aggressiveness of highly metastatic cholangiocarcinoma cells via O-GlcNAcylation. Sci Rep. 2017;7:43842–43842. doi: 10.1038/srep43842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vergara D, Simeone P, Franck J, Trerotola M, Giudetti A, Capobianco L, Tinelli A, Bellomo C, Fournier I, Gaballo A, Alberti S, Salzet M, Maffia M. Translating epithelial mesenchymal transition markers into the clinic: novel insights from proteomics. EuPA Open Proteom. 2016;10:31–41. doi: 10.1016/j.euprot.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singhai R, Patil VW, Jaiswal SR, Patil SD, Tayade MB, Patil AV. E-cadherin as a diagnostic biomarker in breast cancer. N Am J Med Sci. 2011;3(5):227–233. doi: 10.4297/najms.2011.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X, Chen B, Zhu S, Zhang J, He X, Cao G, Chen B. Hyperglycemia promotes Snail-induced epithelial–mesenchymal transition of gastric cancer via activating ENO1 expression. Cancer Cell Int. 2019;19(1):344. doi: 10.1186/s12935-019-1075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu K, Tang Z, Huang A, Chen P, Liu P, Yang J, Lu W, Liao J, Sun Y, Wen S, Hu Y, Huang P. Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and metastasis through upregulation of SNAIL expression. Int J Oncol. 2017;50(1):252–262. doi: 10.3892/ijo.2016.3774. [DOI] [PubMed] [Google Scholar]
- 36.Zaal EA, Berkers CR. The influence of metabolism on drug response in cancer. Front Oncol. 2018;8:500. doi: 10.3389/fonc.2018.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Suo C, Li S-T, Zhang H, Gao P. Metabolic reprogramming for cancer cells and their microenvironment: beyond the Warburg effect. Biochim Biophys Acta. 2018;1870(1):51–66. doi: 10.1016/j.bbcan.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Krasnov GS, Dmitriev AA, Snezhkina AV, Kudryavtseva AV. Deregulation of glycolysis in cancer: glyceraldehyde-3-phosphate dehydrogenase as a therapeutic target. Expert Opin Ther Targets. 2013;17(6):681–693. doi: 10.1517/14728222.2013.775253. [DOI] [PubMed] [Google Scholar]
- 39.Jin L, Zhou Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol Lett. 2019;17(5):4213–4221. doi: 10.3892/ol.2019.10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chunhua Z, Zheng Z, Yuechun Z, Suofu Q. Glucose-6-phosphate dehydrogenase: a biomarker and potential therapeutic target for cancer. Anti Cancer Agents Med Chem. 2014;14(2):280–289. doi: 10.2174/18715206113136660337. [DOI] [PubMed] [Google Scholar]
- 41.Zhang H-S, Zhang Z-G, Du G-Y, Sun H-L, Liu H-Y, Zhou Z, Gou X-M, Wu X-H, Yu X-Y, Huang Y-H. Nrf2 promotes breast cancer cell migration via up-regulation of G6PD/HIF-1α/Notch1 axis. J Cell Mol Med. 2019;23(5):3451–3463. doi: 10.1111/jcmm.14241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lijnen HR, Collen D. Plasminogen: Structure, activation, and regulation. Boston: Springer USA; 2003. Role of the plasminogen and MMP systems in wound healing; pp. 189–200. [Google Scholar]
- 43.Viedma-Rodríguez R, Martínez-Hernández MG, Flores-López LA, Baiza-Gutman LA. Epsilon-aminocaproic acid prevents high glucose and insulin induced-invasiveness in MDA-MB-231 breast cancer cells, modulating the plasminogen activator system. Mol Cell Biochem. 2018;437(1):65–80. doi: 10.1007/s11010-017-3096-8. [DOI] [PubMed] [Google Scholar]
- 44.Caramel J, Ligier M, Puisieux A. Pleiotropic roles for ZEB1 in cancer. Cancer Res. 2018;78(1):30–35. doi: 10.1158/0008-5472.CAN-17-2476. [DOI] [PubMed] [Google Scholar]
- 45.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11(9):670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]