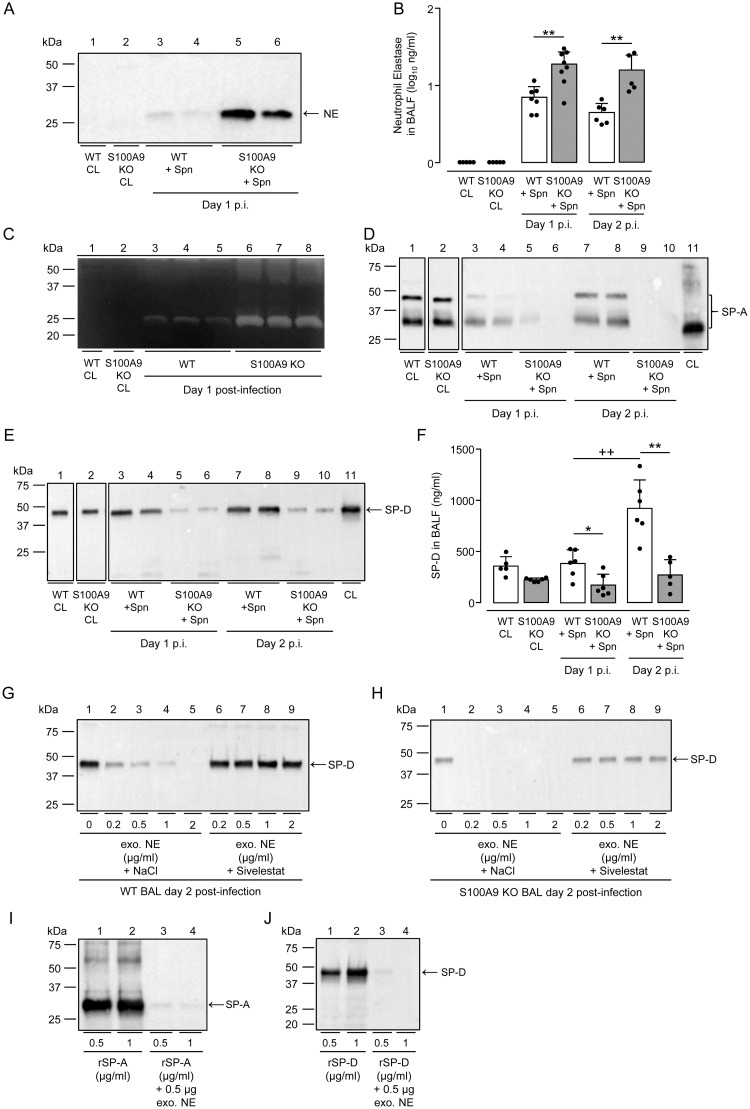
Fig 7. NE dependent degradation of alveolar collectins SP-A and SP-D in the lungs of S. pneumoniae-challenged S100A9 KO mice.
(A) Neutrophil elastase in BAL fluids of untreated or S. pneumoniae-infected WT versus S100A9 KO mice at day 1 post-infection as indicated. Equal amounts of BAL protein (15 μg) were used for western blot analysis. (B) Quantification of NE protein levels in BAL fluids of mice of the respective treatment groups by ELISA. (C) Caseinolytic activity in BALFs of untreated and S. pneumoniae-infected WT and S100A9 KO mice on day 1 post-infection. (D,E) SP-A (D) and SP-D (E) protein levels in BALFs of untreated and S. pneumoniae-challenged WT versus S100A9 KO mice on day 1 and day 2 post-infection, as indicated. Recombinant SP-A or SP-D protein serving as positive control (lane 11 in D,E). (F) Quantification of SP-D protein in BAL fluids of the respective treatment groups. Data are shown as mean ± SD of 5–8 mice per time point and group and are representative of two independently performed experiments. (G,H) Incubation of exogenous NE with BALF of S. pneumoniae-infected WT or S100A9 KO mice led to degradation of SP-D (G, H, lanes 1–5), while pre-incubation of NE with specific inhibitor Sivelestat prevented SP-D degradation in vitro (G,H, lanes 6–9). (I,J) Just 0.5 μg NE are sufficient to degrade rSP-A (I) and rSP-D (J) in vitro. *p ≤ 0.05; **p ≤ 0.01 compared to WT mice, ++p ≤ 0.01 compared to day 2 (Mann-Whitney U test).