Objectives:
The RV144 vaccine trial resulted in a decreased risk of HIV acquisition that was associated with a nonneutralizing antibody response. The objective of this study was to determine the impact of an additional boost to the RV144 vaccine regimen on antibody effector function and durability.
Design:
RV306 was a randomized, double-blind late boosting of the RV144 prime-boost regimen in HIV-uninfected Thai adults (NCT01931358). This analysis included study participants who received the RV144 vaccine regimen and received no additional boost (group 1) or were boosted with ALVAC-HIV and AIDSVAX (group 2) or only AIDSVAX alone (group 3) 24 weeks after completing the RV144 series.
Methods:
Plasma samples from RV306 study participants were used to measure antibody-dependent cellular phagocytosis (ADCP), antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent complement deposition (ADCD), antibody-dependent cellular cytotoxicity (ADCC), trogocystosis, and gp120-specifc IgG subclasses.
Results:
Additional boosting increased the magnitude of all Fc-mediated effector functions 2 weeks following the additional boost compared with 2 weeks after completing the RV144 regimen. However, only trogocytosis remained higher 24–26 weeks after the last vaccination for the study participants receiving an additional boost compared with those that did not receive an additional boost. The additional boost increased IgG1 and IgG4 but decreased IgG3 gp-120 specific antibodies compared with 2 weeks after completing the RV144 regimen.
Conclusion:
Additional boosting of RV144 improved the magnitude but not the durability of some Fc-mediated effector functions that were associated with vaccine efficacy, with trogocytosis being the most durable.
Keywords: antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, antibody-dependent complement deposition, antibody-dependent neutrophil phagocytosis, HIV vaccine, trogocytosis
Introduction
To date, RV144 is the only HIV vaccine to show some overall efficacy in preventing infections in a phase III clinical trial [1]. However, protective efficacy waned from 60% at 12 months to 31% after 3.5 years, suggesting a decline of the protective immune responses. The RV144 vaccine regimen consisted of ALVAC-HIV (vCP1521, a live-attenuated recombinant canarypox virus encoding HIV-1 clade E env, clade B gag, and clade B protease) administered at weeks 0, 4, 12, and 24 together with AIDSVAX B/E gp120 at weeks 12 and 24. Low Env-specific IgA responses or a high IgG/IgA ratio combined with high ADCC responses were identified as a secondary correlate of decreased risk of infection [2]. Studies conducted in nonhuman primates confirmed the efficacy of this regimen and that nonneutralizing functions of antibodies were associated with reduced risk of infections [3–5]. An association between nonneutralizing Fc-mediated effector functions of antibodies and reduced risk of HIV or SIV infection has also been reported for other vaccine regimens using DNA, protein, and, or viral vectors [6–8], therefore, highlighting the importance that these functions may play in preventing HIV infection.
RV306 was a phase I clinical trial that administered the RV144 regimen with an additional boosting consisting of AIDSVAX B/E gp120 alone or together with ALVAC-HIV (vCP1521) [9]. The additional boosting led to improvement in magnitude and durability of CD4+ T-cell responses, binding antibodies, and neutralization. However, the impact of the additional boost on Fc-mediated functions and IgG subclasses has not yet been characterized. Therefore, we sought to determine how antibody-dependent cellular phagocytosis (ADCP), antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent complement deposition (ADCD), trogocytosis, antibody-dependent cellular cytotoxicity (ADCC), and gp120-specifc IgG subclasses were longitudinally impacted by an additional boosting in RV306 study participants.
Materials and methods
Study design and participants
The RV306 clinical trial (NCT01931358) was conducted in healthy Thai volunteers as previously described [9]. Plasma samples from groups 1, 2, and 3 at weeks 0, 26, 50, and 72 were used to measure ADCP, ADNP, ADCD, ADCC, trogocystosis, and gp120-specific IgG subclasses. All plasma samples were run in parallel for ADCP, ADNP, ADCD, and trogocytosis. Effector cells for ADCC, ADNP, and trogocytosis were from one control donor each. Additional methods describing cell lines, primary cells, Fc-mediated effector function assays, and IgG subclasses binding titers are available in the supplementary material.
Statistical analysis
All statistical analysis was performed using Prism, version 9.4.1 for Mac OS (GraphPad Software, La Jolla, California, USA). Paired comparisons within one group were performed using a Wilcoxon test. Comparisons between groups were performed using the Mann–Whitney test. P values below 0.05 were considered significant.
Results
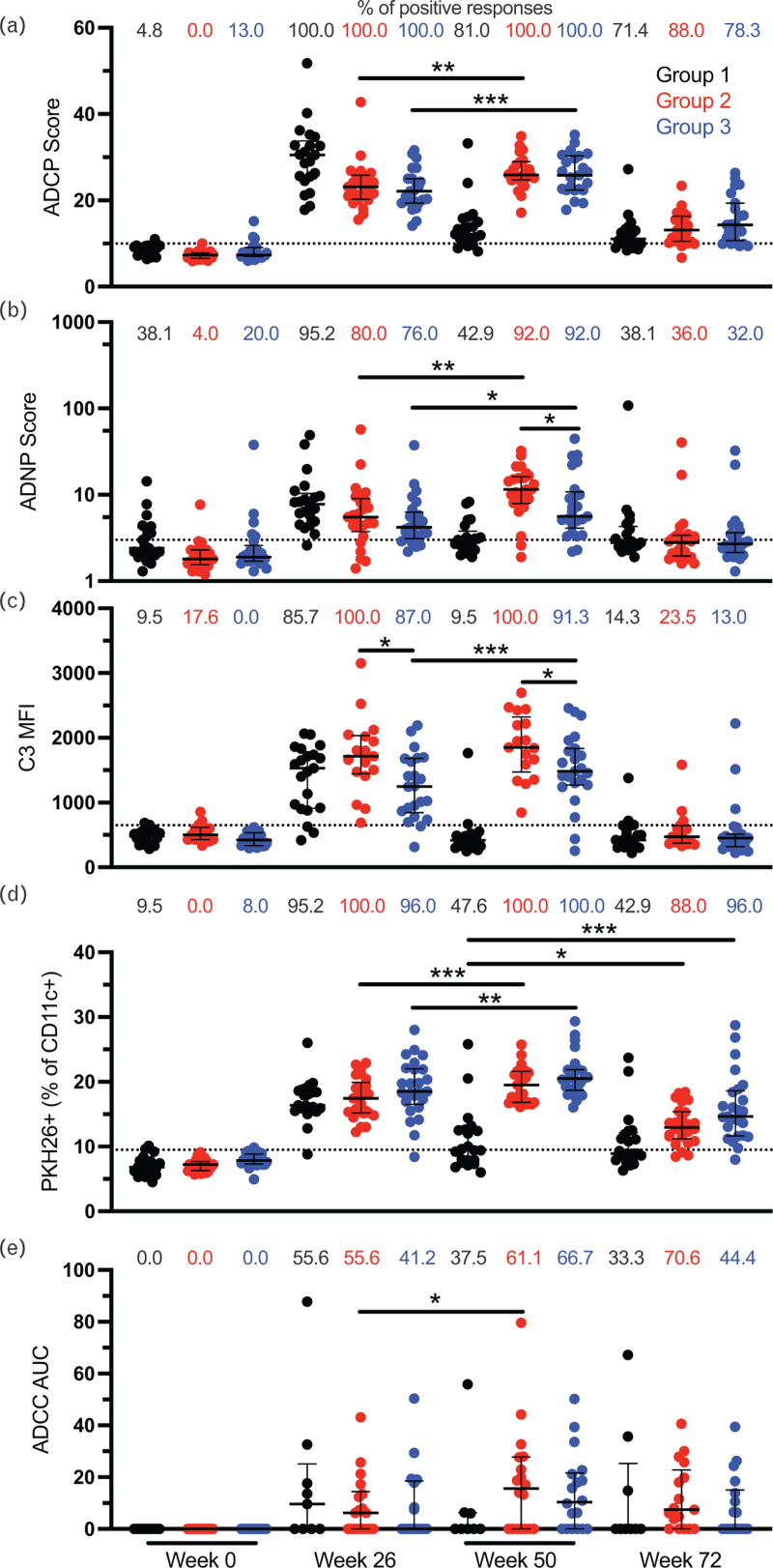
Reduced risk of infection in RV144 was associated with nonneutralizing function of antibodies. However, protection was found to decline rapidly over time. Therefore, we evaluated the impact of an additional boost on the RV144 vaccine regimen on Fc-mediated antibody effector function magnitude and durability using plasma samples from RV306. Groups 2 and 3 received an additional vaccination at week 48 consisting of ALVAC-HIV (vCP1521) together with AIDSVAX B/E gp120 or AIDSVAX B/E gp120 alone, respectively, and group 1 consisted of the RV144 vaccine regimen without additional boosting (Supplemental Figure 1). ADCP, ADNP, ADCD, ADCC, and trogocytosis were evaluated longitudinally at weeks 0 (baseline), 26 (RV144 peak immunogenicity), 50 (2 weeks post additional boost), and 72 (24 weeks post additional boost). All Fc-mediated functions were increased at week 50 compared with week 26 for both groups 2 and 3 except for ADCD in group 2 and ADCC in group 3 (Fig. 1a–e). All functions declined by week 72, although most study participants from groups 2 and 3 maintained detectable ADCP and trogocytosis (Fig. 1a and d). There was no difference in the magnitude of the Fc-mediated functions between groups 2 and 3 after the additional boost (week 50) except for ADCD and ADNP (Fig. 1b and c). Group 2 participants, receiving both ALVAC-HIV (vCP1521) and AIDSVAX B/E gp120, had higher ADCD and ADNP compared with those of group 3. For ADCD, there was a significant difference between groups 2 and 3 already at week 26 (Fig. 1c). There were no significant differences between groups 2 and 3 at week 72 for all functions.
Fig. 1.
Peak levels of Fc-mediated effector function are increased following an additional boost to the RV144 vaccine regiment.
ADCP (a), ADNP (b), ADCD (c), trogocytosis (d), and ADCC (e) were measured at weeks 0, 26, 50, and 72 in RV306 study participants. Black, red, and blue represent groups 1, 2, and 3 respectively. For ADNP and trogocystosis, N = 21, 25, and 25 for groups 1, 2, and 3 respectively. For ADCP, N = 21, 25, and 23 for groups 1, 2, and 3, respectively. For ADCD, N = 21, 17, and 23 for groups 1, 2, and 3, respectively. For ADCC, N = 9, 18, and 18 for groups 1, 2, and 3, respectively. The percentage of positive responses at each time point for each group is indicated at the top of each graph. Dotted lines indicate the cutoff for positivity. Error bars represent the median and interquartile range, respectively. ADCD, antibody-dependent complement deposition; ADCP, antibody-dependent cellular phagocytosis; ADNP, antibody-dependent neutrophil phagocytosis. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Next, we evaluated the impact of an additional boost on the durability of the Fc-mediated effector functions 24–26 weeks after the last vaccination by comparing groups 2 and 3 at week 72 to group 1 (no additional boost) at week 50. There was no difference in ADCP, ADNP, ADCD, and ADCC between the groups (Fig. 1a–c and e). However, study participants receiving an additional boost (groups 2 and 3) had higher trogocytosis compared with those that did not (group 1) (Fig. 1d).
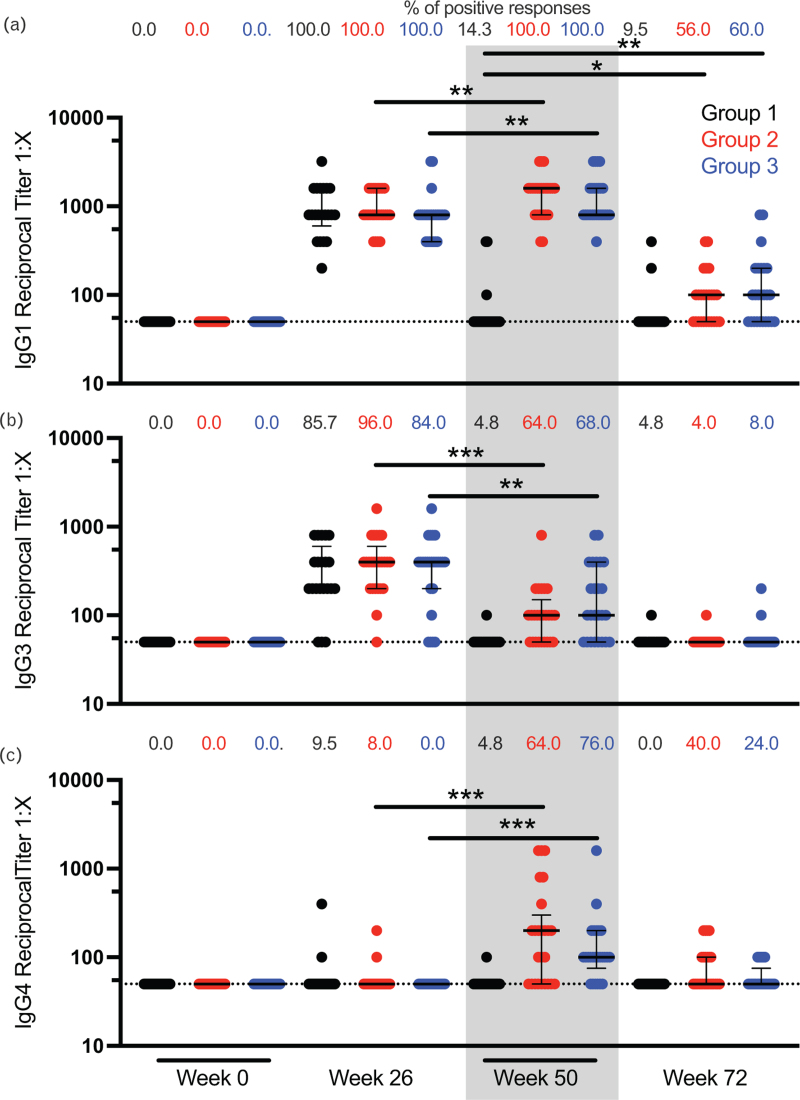
Finally, we measured the gp120-specific binding titer for IgG subclasses longitudinally in RV306 participants. IgG1 and IgG4 titers were increased after an additional boost at week 50 compared with week 26 for groups 2 and 3 (Fig. 2a and c). On the opposite, IgG3 titers were decreased at week 50 compared with week 26 for participants that received an additional boost (Fig. 2b). No IgG2 binding was detected at any time point (data not shown). IgG1 durability was increased with the additional boost when comparing groups 2 and 3 at week 72 to group 1 at week 50 (Fig. 2a).
Fig. 2.
IgG subclass binding titer to gp120.
IgG1 (a), IgG3 (b), and IgG4 (c) antibody-binding titers to HIV-1 gp120 A244 were measured by ELISA at weeks 0, 26, 50, and 72 in RV306 study participants. Black, red, and blue represent groups 1 (N = 21), 2 (N = 25), and 3 (N = 25), respectively. Each symbol represents the average of triplicate measurements for a participant. The percentage of positive responses at each time point for each group is indicated at the top of each graph. Dotted lines indicate the cutoff for positivity. Error bars represent the median and interquartile range, respectively. The gray rectangle highlights the increase in IgG4 and decrease in IgG3 at week 50. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Discussion
One possible strategy to improve the durability of the RV144 vaccine regimen is additional boosting vaccinations. Here, we specifically looked at the effect of an additional boost on Fc-mediated antibody effector functions, some of which have been associated with reduced risk of infection in clinical trials or in animal models. Previous work using cross-sectional samples evaluated the impact of two additional boosts on the RV144 vaccine regimen with an interval of 6–8 years and found that the additional boosts restored ADCP back to RV144 peak levels but increased the peak magnitude of other Fc-mediated functions [10]. Here, using longitudinal samples, we report that ADCP, similar to other Fc-mediated functions, is also increased by an additional vaccination 24 weeks later. Similarly to the same previous study [10], we found that an additional boost increased both IgG1 and IgG4 but decreased IgG3. A similar pattern for IgG3 and IgG4 was also reported in the VAX003 and VAX004 trials that included additional protein boosts compared with RV144 [11]. This may be important because the functional profile varies between the IgG subclasses. IgG3 has been associated with lower risk of infection in RV144 [12] and high Fc-mediated antibody polyfunctionality while IgG4 has been negatively associated with antibody functionality [13]. Inclusion of ALVAC-HIV (vCP1521) in the boost did not impact the levels of ADCP, trogocytosis or ADCC at the peak immunogenicity time point (week 50) but increased the levels of ADNP and ADCD. However, the levels of ADNP and ADCD were mostly undetectable for both groups 24 weeks post vaccination, suggesting that, if there is an additive effect of ALVAC-HIV (vCP1521) on those functions, it is short lived. In fact, only monocyte-mediated functions, ADCP and trogocytosis, remained detectable in most study participants 24–26 weeks after the last vaccination, indicating that activation of monocytes may have a lower threshold than activation of neutrophils and natural killer (NK) cells. This could be due in part to differences in expression of Fcγ receptors (FcγR) between the cell types as well as differences in affinity between the FcγR. HVTN702 tested ALVAC-HIV and bivalent gp120 subtype C together with MF59 and included additional boosts compared with RV144 but did not show efficacy [14]. This observation further highlights that strategies other than additional boosting will be needed to improve the durability of ADCC that was identified as a secondary correlate of reduced HIV acquisition risk in RV144. Delayed boosting, novel adjuvants, and novel antigens are some of the possible strategies that could be tested. Interestingly, trogocytosis was the only function in which both peak magnitude and durability were increased by an additional boost. Several functions have been associated with trogocytosis including immune evasion of target cells, increased antigen presentation by the effector cells, and target cell killing (reviewed in [15]). One study using plasma samples from people with HIV suggested that trogocytosis could reduce the viability of target cells and was associated with the development of broadly neutralizing antibodies [16]. More investigations are needed to understand the role of trogocytosis in the control of viral infections. Finally, understanding the determinants of vaccine durability would be beneficial for all HIV vaccine strategies.
Acknowledgements
We are deeply grateful to the RV306 participants for their participation and dedication to this study, as well as our Community Advisory Boards for their helpful input.
Contributors: N.P., P.P., S.N., S.G., F.S., J.A.A., R.J.O., and S.V. designed the study. N.P., P.P., S.N., and S.C. conducted the clinical studies. D.P.P., M.A.E., and Z.S. did the ADCP, ADNP, ADCD, and trogocytosis assays. S.S.O. and G.F. did the ADCC assay. J.P. and S.A. did the IgG subclass binding titer. D.P.P., S.S.O., and Z.S. conducted data analysis. D.P.P., S.V., and G.F. and wrote the manuscript. All authors reviewed and edited the manuscript.
Funding: This work was supported by the US Army Medical Research and Development Command (Military Infectious Diseases Research Program; cooperative agreement W81XWH-18-2-0040 with the Henry M Jackson Foundation for the Advancement of Military Medicine), the US Army Medical Materiel Development Activity, and the National Institute of Allergy and Infectious Diseases (interagency agreement Y1-AI-2642-12 with the US Army Medical Research and Materiel Command).
Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the US Army, the Department of Defense, nor the Henry M Jackson Foundation for the Advancement of Military Medicine. The investigators have adhered to the policies for protection of human participants as prescribed in AR 70-25. Trade names are used for identification purposes only and do not imply endorsement. This article was prepared while Michael A. Eller was employed at Henry M. Jackson Foundation for the Advancement of Military Medicine for the U.S. Military HIV Research Program.
Conflicts of interest
S.G. is an employee and shareholder of Sanofi Pasteur. F.S. is an employee of Global Solutions for Infectious Diseases. All other authors declare no conflicts of interest.
Supplementary Material
Current affiliation: U.S. Food and Drug Administration, MD, USA.
Current affiliation: Vaccine Research Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA.
The RV306 Study Group members are listed at.
Supplemental digital content is available for this article.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–2220. [DOI] [PubMed] [Google Scholar]
- 2.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorini G, Fourati S, Vaccari M, Rahman MA, Gordon SN, Brown DR, et al. Engagement of monocytes, NK cells, and CD4+ Th1 cells by ALVAC-SIV vaccination results in a decreased risk of SIVmac251 vaginal acquisition. PLoS Pathog 2020; 16:e1008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaccari M, Fourati S, Gordon SN, Brown DR, Bissa M, Schifanella L, et al. HIV vaccine candidate activation of hypoxia and the inflammasome in CD14(+) monocytes is associated with a decreased risk of SIV(mac251) acquisition. Nat Med 2018; 24:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, et al. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat Commun 2017; 8:15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neidich SD, Fong Y, Li SS, Geraghty DE, Williamson BD, Young WC, et al. Antibody Fc effector functions and IgG3 associate with decreased HIV-1 risk. J Clin Invest 2019; 129:4838–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alter G, Yu WH, Chandrashekar A, Borducchi EN, Ghneim K, Sharma A, et al. Passive transfer of vaccine-elicited antibodies protects against SIV in rhesus macaques. Cell 2020; 183:185.e14–196.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Om K, Paquin-Proulx D, Montero M, Peachman K, Shen X, Wieczorek L, et al. Adjuvanted HIV-1 vaccine promotes antibody-dependent phagocytic responses and protects against heterologous SHIV challenge. PLoS Pathog 2020; 16:e1008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitisuttithum P, Nitayaphan S, Chariyalertsak S, Kaewkungwal J, Dawson P, Dhitavat J, et al. RV306 study group. Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial. Lancet HIV 2020; 7:e238–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischinger S, Shin S, Boudreau CM, Ackerman M, Rerks-Ngarm S, Pitisuttithum P, et al. Protein-based, but not viral vector alone, HIV vaccine boosting drives an IgG1-biased polyfunctional humoral immune response. JCI Insight 2020; 5:e135057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnasuta C, Akapirat S, Madnote S, Savadsuk H, Puangkaew J, Rittiroongrad S, et al. Comparison of antibody responses induced by RV144, VAX003, and VAX004 vaccination regimens. AIDS Res Hum Retroviruses 2017; 33:410–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 2014; 6:228–238. [DOI] [PubMed] [Google Scholar]
- 14.Gray GE, Bekker LG, Laher F, Malahleha M, Allen M, Moodie Z, et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in Adults. N Engl J Med 2021; 384:1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao S, Zhang L, Xiang S, Hu Y, Wu Z, Shen J. Gnawing between cells and cells in the immune system: friend or foe? A review of trogocytosis. Front Immunol 2022; 13:791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson SI, Crowther C, Mkhize NN, Morris L. Measuring the ability of HIV-specific antibodies to mediate trogocytosis. J Immunol Methods 2018; 463:71–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.