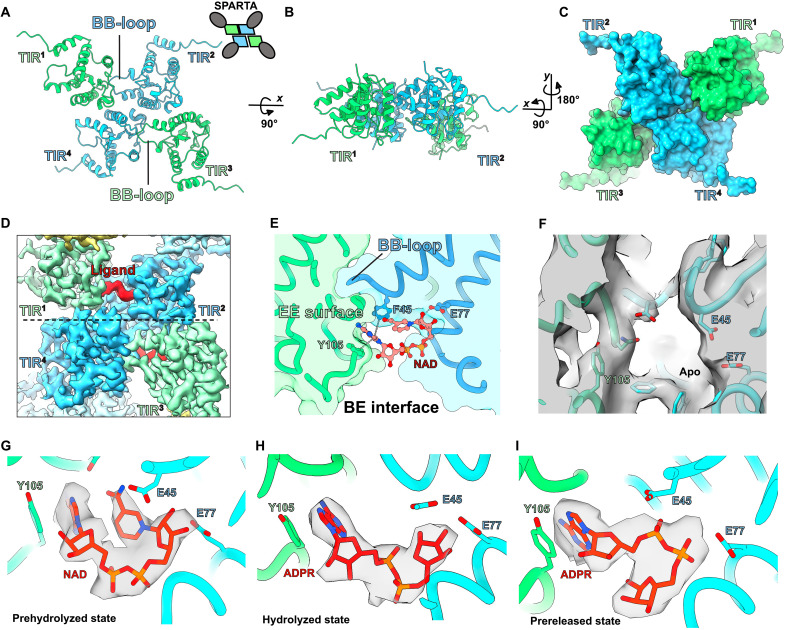
Fig. 4. Snapshots of the M. polysiphoniae SPARTA (MapSPARTA) NADase activity.
(A to C) The structural arrangement of toll/interleukin-1 receptor/resistance protein (TIR) domains from the MapSPARTA tetramer. (D) Cryo–electron microscopy (cryo-EM) density of a ligand/substrate bound (in red) in the TIR domains (in green and blue). (E) Details of the interaction between the bound nicotinamide adenine dinucleotide (oxidized form) (NAD) and residues of the TIR domain. (F) Cryo-EM density showing empty ligand/substrate binding pocket in apo MapSPARTA tetramer (G to I) Cryo-EM density for NAD and adenosine 5´-diphosphoribose (ADPR) in the prehydrolyzed, hydrolyzed, and prereleased states.