Abstract
Background
It has been reported that the hepatic vein waveforms determined by abdominal ultrasonography can assess hepatic congestion in patients with heart failure (HF). However, the parameter that quantifies hepatic vein waveforms has not been established. We suggest the hepatic venous stasis index (HVSI) as the novel indicator to evaluate hepatic congestion quantitatively. To examine the clinical significance of HVSI in patients with HF, we aimed to clarify the associations of HVSI with the parameters of cardiac function and right heart catheterization, as well as that with prognosis, in patients with HF.
Methods and Results
We performed abdominal ultrasonography, echocardiography, and right heart catheterization in patients with HF (n=513). The patients were divided into 3 groups based on HVSI as follows: HVSI 0 (HVSI=0, n=253), low HVSI (HVSI 0.01–0.20, n=132), and high HVSI (HVSI>0.20, n=128). We examined the associations of HVSI with parameters of cardiac function and right heart catheterization and followed up for cardiac events defined as cardiac death or worsening HF. There was a significant increase in level of B‐type natriuretic peptide, inferior vena cava diameter, and mean right atrial pressure with increasing HVSI. During the follow‐up period, cardiac events occurred in 87 patients. In the Kaplan–Meier analysis, cardiac event rate increased across increasing HVSI (log‐rank, P=0.002).
Conclusions
HVSI assessed by abdominal ultrasonography reflects hepatic congestion and right‐sided HF and is associated with adverse prognosis in patients with HF.
Keywords: heart failure, hemodynamics, hepatic vein, liver, prognosis
Subject Categories: Heart Failure
Nonstandard Abbreviations and Acronyms
- AHEAD score
long‐term risk classification in acute heart failure
- CVP
central venous pressure
- HV
hepatic vein
- HVSI
hepatic venous stasis index
- IRVF
intrarenal venous flow
- RAP
right atrial pressure
- RHC
right heart catheterization
- RVSI
renal venous stasis index
- SWD
shear wave dispersion
- SWE
shear wave elastography
Clinical Perspective.
What Is New?
Hepatic venous stasis index assessed by abdominal ultrasonography is a novel indicator that is able to evaluate hepatic congestion quantitatively.
What Are the Clinical Implications?
Hepatic venous stasis index reflected hepatic congestion and right‐sided heart failure.
High hepatic venous stasis index was associated with adverse prognosis in patients with heart failure.
Heart failure (HF) is a refractory clinical syndrome that originates from various types of structural or functional heart diseases and is a major public health problem that leads to frequent hospitalizations, impaired quality of life, and shortened life expectancy. 1 , 2 The number of patients with HF has been rapidly increasing to an estimated 26 million patients worldwide. 3 , 4 , 5 , 6 The elderly often have multiple comorbidities, including hypertension, atrial fibrillation (AF), chronic kidney disease (CKD), chronic obstructive pulmonary disease, and anemia, all of which aggravate HF and affect prognosis. 7 Renal dysfunction and liver dysfunction are common adverse outcome predictors in HF. 8 , 9 The cardio‐hepatic syndrome can occur because of cardio‐hepatic interactions including the development of congestive hepatopathy (passive congestion) and hypoxic hepatitis (reduced arterial perfusion) in patients with HF. 1 , 7 , 8 , 10 , 11 , 12 , 13 Congestion is a key feature in HF, and its presence is associated with poor prognosis. 14 Invasive tests such as right heart catheterization (RHC) have been used to objectively evaluate the degree of congestion because sometimes it is not clinically evident. 15 , 16 Recently, abdominal ultrasonography has been suggested as a form of noninvasive image testing. 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28
Regarding abdominal ultrasonography for assessing congestion, it has recently been reported that the intrarenal venous flow (IRVF) patterns assessed by renal Doppler ultrasonography and the renal venous stasis index (RVSI) quantifying IRVF reflect renal congestion and are associated with adverse prognosis in patients with HF. 17 , 18 , 21 , 23 , 24 Concerning hepatic congestion, it has been reported that liver stiffness measured by shear wave elastography (SWE) is an indicator of hepatic congestion due to increased right‐sided filling pressure (ie, central venous pressure [CVP]). 19 , 25 , 26 , 27 , 28 In addition, it has been reported that shear wave dispersion (SWD) may reflect remaining fibrosis after improvement of congestion. 20 IRVF, RVSI, SWE, and SWD are associated with adverse prognosis in patients with HF. On the other hand, it has been reported that the hepatic vein (HV) waveforms can be used to assess hepatic congestion. 22 The parameters that quantify HV waveforms have not been established. We suggest the hepatic venous stasis index (HVSI) as a new indicator to evaluate hepatic congestion quantitatively.
To examine the clinical significance of HVSI in patients with HF, we aimed to clarify the associations of HVSI with laboratory tests, echocardiography, and RHC, as well as its prognostic impact in patients with HF.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects and Protocol
Figure 1 shows a patient flow diagram. This was a prospective observational study of 556 patients who were classified as either stage C or stage D of HF stage classification in the American College of Cardiology Foundation/ American Heart Association guidelines and were hospitalized and underwent abdominal ultrasonography in Fukushima Medical University Hospital between April 2018 and September 2021. 29 Treatment of decompensated HF was provided by each patient's attending cardiologist based on the established HF guidelines. 29 , 30 , 31 , 32 Blood samples, abdominal ultrasonography, echocardiography, and RHC were obtained during hospitalization from patients in a stable condition before discharge. A total of 556 patients underwent abdominal ultrasonography, all of whom underwent testing for hepatitis B surface antigen and hepatitis C antibodies, and their medical histories were checked for chronic liver disease (eg, cirrhosis, hepatic tumors, bile duct disease) and alcohol abuse (≥30 g/day for men, ≥20 g/day for women). Any patients who had the aforementioned liver diseases (n=32) or were undergoing dialysis (n=11) were excluded from the study. Finally, 513 patients were enrolled, among whom 310 had undergone RHC. Patients' baseline data were collected at discharge. Abdominal ultrasonography was performed during hospitalization in patients in a stable condition without changes in medications and the duration from baseline data collection to abdominal ultrasonography was median 4 days. Echocardiography and RHC were performed within 3 days of abdominal ultrasonography. Laboratory test was performed on the same day as RHC. Of these patients, those with HVSI of 0 were classified as the HVSI 0 group (HVSI=0, n=253, 49%). Because HVSI is our original and novel indicator, there is no common cutoff value. Therefore, patients with HVSI above 0 were divided into 2 groups based on the median HVSI value (0.20) in the present population: the low HVSI group (HVSI 0.01–0.20, n=132, 26%) and the high HVSI group (HVSI >0.20, n=128, 25%). These patients were also classified into 3 categories based on their HV waveforms: those with a continuous pattern (C group), those whose V wave ran under the baseline (U group), and those with a reversed V wave (R group). 22
Figure 1. Patient flow diagram.
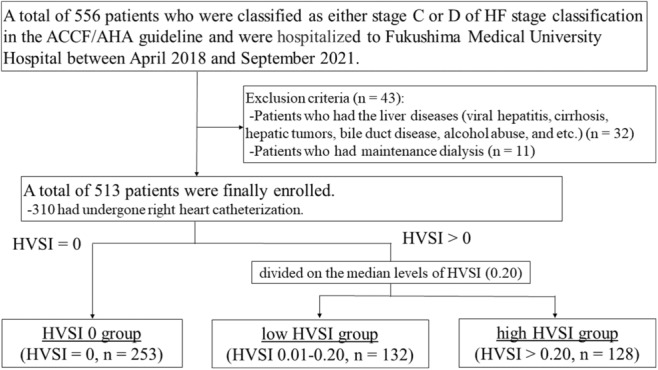
A total of 556 patients who were classified as either stage C or D of HF stage classification in the ACCF/AHA guidelines and were hospitalized at Fukushima Medical University Hospital between April 2018 and September 2021 underwent abdominal ultrasonography. Patients who had chronic liver disease (eg, viral hepatitis, cirrhosis, hepatic tumors, bile duct disease, alcohol abuse) or were undergoing dialysis were excluded from the study. Finally, 513 patients were enrolled, among whom 310 had undergone right heart catheterization. Of these patients, those with HVSI of 0 were classified as the HVSI 0 group. Patients with HVSI above 0 were divided into 2 groups based on the median HVSI value (0.20) in the present population: the low HVSI group and the high HVSI group. ACCF indicates American College of Cardiology Foundation; AHA, American Heart Association; HF, heart failure; and HVSI, hepatic venous stasis index.
First, we compared the clinical features and the results from laboratory tests, echocardiography, and RHC among the 3 groups. Second, the patients were followed up until March 2022 for cardiac events as composites of cardiac death or worsening HF. Cardiac death was defined as death from lethal arrhythmia such as ventricular fibrillation, acute coronary syndromes, or worsening HF. Worsening HF was defined as unplanned rehospitalization for HF treatment. For patients who experienced ≥2 events, only the first event was included in the analysis. We were able to follow up with all patients because they all visited the hospital monthly or every other month. Status and dates of death were obtained from the patients' medical records. Third, we evaluated prognostic value of HVSI. We calculated the AHEAD (A–atrial fibrillation, H–hemoglobin levels of <12.0 g/dL in women and <13.0 g/dL in men [anemia], E–elderly [age >70 years], A–abnormal renal parameters [creatinine>130 μmol/L], D–diabetes) score, which was known as predictive model for HF. Each risk factor of the AHEAD score was counted as 1 point. 33 Then, we created a modified AHEAD score by adding HVSI to the AHEAD score and tested the improvement in prediction. Those administering the survey were blind to the analyses, and written informed consent was obtained from all study subjects. The study protocol was approved by the Ethics Committee of Fukushima Medical University (No. 823) and was carried out in accordance with the principles outlined in the Declaration of Helsinki. Reporting of the study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and the Enhancing the Quality and Transparency of Health Research guidelines.
Ischemic coronary artery disease was confirmed by either past medical history or by myocardial scintigraphy, coronary computed tomography angiography, or coronary angiography. AF was confirmed by ECG performed during hospitalization or from medical records including past medical history. Hypertension was defined as a systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or use of antihypertensive drugs. 34 Dyslipidemia was defined as triglyceride levels of ≥150 mg/dL, low‐density lipoprotein cholesterol levels of ≥140 mg/dL, high‐density lipoprotein cholesterol levels of <40 mg/dL, or use of cholesterol‐lowering drugs. 35 Diabetes was defined as the recent use of antidiabetic drugs, fasting glucose levels of ≥126 mg/dL, casual glucose levels of ≥200 mg/dL, or glycated hemoglobin levels of ≥6.5% (National Glycohemoglobin Standardization Program). 36 CKD was defined as estimated glomerular filtration rate of <60 mL/min per 1.73 m2. 37 , 38 , 39 Anemia was defined as hemoglobin levels of <12.0 g/dL in women and <13.0 g/dL in men. 40 , 41
Abdominal Ultrasonography
The actual methods of acquisition were as follows. Two experienced sonographers (M.M., with 30 years of experience in abdominal ultrasonography, and S.I., with 20 years of experience) performed abdominal ultrasonography, using an Aplio i800 system (Canon Medical Systems Corporation, Tochigi, Japan) with a 1.8 to 6.4 MHz convex transducer. The velocity range of the color Doppler was set to approximately 10 to 20 cm/s. The 2 examiners were blinded to all clinical data. The patients fasted for at least 12 hours before the abdominal ultrasonography and were placed in the supine position. The transducer was placed in the intercostal or subcostal space, and the patient's right arm was raised above the head to obtain a proper acoustic window. The tip of the probe transducer was placed perpendicular to the liver capsule to avoid refraction of the acoustic radiation force impulse. HV was identified in reference to continuity with the inferior vena cava, direction of blood flow, and other identifiers. The measurements were undertaken by approaching the HV 3 to 5 cm proximal to the inferior vena cava from the right intercostal space. We selected the right HV because it was detected easily. The angle of the ultrasound beam to right HV blood flow was less than 60°. A gray‐scale B‐mode evaluation of the liver was first performed, followed by color and spectral Doppler evaluation. As shown in Figure 2, HV waveforms were obtained using a pulsed‐wave Doppler device. The Doppler gate was placed in nonforced end inspiration. The downward Doppler signal indicates the venous return flow (antegrade flow), the upward Doppler signal indicates the reversed blood flow to the liver from the heart (retrograde flow), and no signal indicates stasis flow. We defined the total of reversed blood flow time to the liver from the heart and stasis flow time as HV stasis flow time. In reference to calculation of RVSI, 21 , 42 we calculated HVSI, which indicates the proportion of the HV statis flow time to the HV waveform cycle time, as follows: HV stasis flow time/HV waveform cycle time. In patients with sinus rhythm, we selected the most stable value among 5 HV waveform cycles. In patients with AF, an index beat (the beat following 2 preceding cardiac cycles of equal duration) was used for each measurement. When the venous return flow shows continuous flow, the cardiac cycle time was used as the HV waveform cycle time. Next, the maximal flow velocity (V), ventricular systolic (S), ventricular diastolic (D), and atrial reversal (A) waves were measured. We classified HV waveforms into 3 groups based on previous studies by the shape and position of the V wave: those in whom the continuous flow pattern or V wave was ambiguous (C group), those in whom the V wave ran under the baseline (U group), and those who had a reversed V wave (R group). 43 , 44 , 45 , 46 Figure 2 shows representative HVSI along with HV waveform groups. Figure 2A, HVSI=0 with C group; Figure 2B, HVSI=0 with C group; Figure 2C, low HVSI with C group; Figure 2D, HVSI=0 with U group; Figure 2E, low HVSI with U group; Figure 2F, high HVSI with R group; and Figure 2G, high HVSI with R group. Although the R group absolutely shows HVSI>0, the C and U groups sometimes show HVSI=0, and sometimes HVSI>0. We measured aorta preceliac peak systolic velocity, aorta preceliac velocity time integral, SWE, SWD, RVSI, and IRVF patterns, based on previous studies. 17 , 19 , 20 , 21 In addition, we divided patients into 3 groups based on the median value of SWE (1.36): the low SWE group (SWE ≤1.36, n=246) and the high SWE group (SWE >1.36, n=250). We also divided patients into 3 groups based on the median value of SWD (10.0): the low SWD group (SWD ≤10.0, n=189) and the high SWD group (SWD >10.0, n=177).
Figure 2. Abdominal ultrasonography.
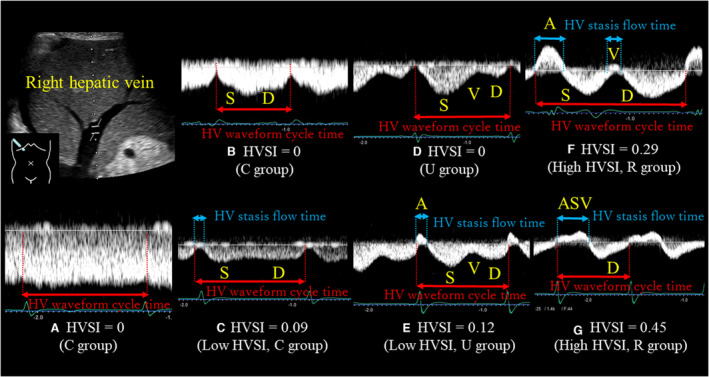
All abdominal ultrasonography studies were performed in the right HV. HV waveforms were obtained using a pulsed‐wave Doppler device. The downward Doppler signal indicates the venous return flow (antegrade flow), the upward Doppler signal indicates the reversed blood flow to the liver from the heart (retrograde flow), and no signal indicates stasis flow. We defined the total of reversed blood flow time to the liver from the heart and stasis flow time as HV stasis flow time. We calculated HVSI, which indicates the proportion of the HV statis flow time to the HV waveform cycle time, as follows: HV stasis flow time/HV waveform cycle time. Patients with an HVSI of 0 were defined as the HVSI 0 group (HVSI=0). Patients with HVSI above 0 were divided into 2 groups based on the median value of HVSI (0.20): the low HVSI group (HVSI 0.01–0.20) and the high HVSI group (HVSI>0.20). Next, the maximal flow velocity (V), ventricular systolic (S), ventricular diastolic (D), and atrial reversal (A) waves were measured. We classified HV waveforms into 3 groups based on previous studies by the shape and position of the V wave: those in whom the continuous flow pattern or V wave was ambiguous (C group), those in whom the V wave ran under the baseline (U group), and those who had a reversed V wave (R group). HVSI increased with increasing severity of congestion. This figure shows representative HVSI and HV waveform groups in patients with HF. A, HVSI=0 with C group. B, HVSI=0 with C group. C, Low HVSI with C group. D, HVSI=0 with U group. E, Low HVSI with U group. F, High HVSI with R group. G, High HVSI with R group. HV indicates hepatic vein; and HVSI, hepatic venous stasis index.
Echocardiography
Echocardiography was performed by experienced echocardiographers based on current recommendations. 47 The echocardiographic parameters included left ventricular ejection fraction (LVEF), left ventricular outflow tract velocity time integral, left atrial volume, early trans mitral flow velocity to mitral annular velocity ratio, right atrium area, right ventricular areas, right ventricular fractional area change, inferior vena cava diameter, severity of tricuspid regurgitation, tricuspid regurgitation pressure gradient, tricuspid annular plane systolic excursion, and tissue Doppler‐derived tricuspid lateral annular systolic velocity. 48 , 49 The LVEF was calculated using Simpson's method. The right ventricular fractional area change, defined as [right ventricular end diastolic area−right ventricular end systolic area]/right ventricular end diastolic area×100, is a measure of right ventricular systolic function. 50 All measurements were performed using ultrasound systems (ACUSON Sequoia, Siemens Medical Solutions USA, Inc., Mountain View, CA).
Right Heart Catheterizations and Hemodynamics
Of all 513 patients, 310 underwent RHC based on remedial judgment of the attending physician. All RHCs were performed under fluoroscopic guidance with patients in the resting supine position breathing room air at rest. Mean right atrial pressure (RAP), mean pulmonary artery pressure, mean pulmonary artery wedge pressure, cardiac index, and pulmonary vascular resistance (PVR) were measured using a 6F Swan Ganz catheter (Edwards Lifesciences, Irvine, CA). CI was calculated based on the thermodilution method. 51
Measurement of Laboratory Data
We measured BNP (B‐type natriuretic peptide) levels using a specific immunoradiometric assay (Shionoria BNP kit, Shionogi, Osaka, Japan). This assay was performed by experienced laboratory technicians who were blinded to characteristics of patients.
Statistical Analysis
Normally distributed data were presented as mean±SD, and nonnormally distributed data were presented as median and interquartile range (ie, median [25th percentile–75th percentile]). Categorical variables were expressed as numbers and percentages, and the chi‐square test was used for their comparisons. Among the 3 groups, parametric variables were compared using 1‐way ANOVA, and nonparametric variables were compared using the Kruskal–Wallis test. Kaplan–Meier analysis with log‐rank test was used to evaluate the cardiac event rate. HVSI was assessed as a predictor of postdischarge cardiac events by the Cox proportional hazard analysis. In addition, the statistical tests based on the Schoenfeld residuals which can assess the correlation between scaled residuals and time, was also conducted for a proportional hazard assumption. In the Cox proportional hazard analysis, to prepare for potential confounding, we considered the following clinical factors: age, sex, body mass index and confounding factors that were generally recognized as poor prognostic factors (ie, diabetes, CKD, AF, anemia, LVEF, and BNP). Univariate factors with P<0.05 were entered into the multivariable Cox proportional hazard analyses. We fit a Cox regression model with the calculated AHEAD score as the only independent variable, and we calculated the c‐index, which is regarded as a measure for model discrimination equivalent to the area under the curve for binary dependent variables. Receiver operating characteristic curve was computed by using the AHEAD score as the reference variable and the cardiac events as the binary reference variable. The modified AHEAD score was created by adding HVSI as a covariate and incorporated along with the AHEAD score as independent variables and cardiac events as the dependent variable. Model improvement was assessed by the regression method. A value of P<0.05 was defined as statistically significant for all comparisons. All statistical analyses were performed using Statistical Package for Social Science version 27.0 (IBM, Armonk, NY) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Table 1 shows the clinical and demographic characteristics of the study population. Of the 513 patients included in the study, the median age was 73.0 (63.0–81.0) years, and 289 (56.3%) were male. The breakdown of angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor neprilysin inhibitor was 203 (39.6%) with angiotensin‐converting enzyme inhibitor, 138 (26.9%) with angiotensin receptor blocker, and 0 (0%) with angiotensin receptor neprilysin inhibitor. During the study period, angiotensin receptor neprilysin inhibitor had not been approved in Japan. The percentage of patients who show New York Heart Association class III or IV, the prevalence of AF, and usage of mineralocorticoid receptor antagonist and diuretic increased in proportion to HVSI. In contrast, age, sex, body mass index, pathogenesis, and the prevalence of hypertension, dyslipidemia, diabetes, CKD, anemia, and other medication did not differ among the groups.
Table 1.
Clinical and Demographic Characteristics of the Study Population
| Total (n=513) | HVSI=0 (HVSI=0, n=253) | Low HVSI (HVSI 0.01–0.20, n=132) | High HVSI (HVSI>0.20, n=128) | P value* | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 73.0 (63.0–81.0) | 72.0 (62.0–80.0) | 76.0 (68.0–82.0) | 71.0 (59.0–79.0) | 0.007 |
| Male sex, n (%) | 289 (56.3) | 148 (58.5) | 71 (53.8) | 70 (54.7) | 0.616 |
| Body mass index, kg/m2 | 22.6 (20.3–25.4) | 22.8 (20.6–25.6) | 22.2 (19.9–24.8) | 22.7 (20.2–25.1) | 0.265 |
| Systolic blood pressure, mm Hg | 118.0 (106.0–131.0) | 120.0 (107.0–131.0) | 122.0 (109.0–137.0) | 110.0 (100.3–125.8) | <0.001 |
| Diastolic blood pressure, mm Hg | 66.0 (59.0–75.0) | 66.0 (59.5–76.0) | 65.0 (58.0–75.0) | 67.0 (58.0–74.0) | 0.570 |
| Heart rate, bpm | 69.0 (61.0–81.0) | 70.0 (61.0–82.0) | 68.0 (60.0–77.0) | 70.0 (62.0–84.8) | 0.122 |
| New York Heart Association class III or IV, n (%) | 167 (33.9) | 68 (28.6) | 41 (31.8) | 58 (46.0) | 0.003 |
| Etiology, n (%) | |||||
| ischemic/myopathy/valvular/arrhythmia/pulmonary/congenital/others | 101 (19.7)/126 (24.6)/178 (34.7)/48 (9.4)/41 (8.0)/8 (1.6)/11 (2.1) | 56 (22.1)/57 (22.5)/90 (35.6)/21 (8.3)/21 (8.3)/4 (1.6)/4 (1.6) | 28 (21.2)/35 (26.5)/48/(36.4)/8 (6.1)/7 (5.3)/2 (1.5)/4 (3.0) | 17 (13.3)/34 (26.6)/40 (31.3)/19 (14.8)/13 (10.2)/2 (1.6)/3 (2.3) | 0.311 |
| Comorbidities | |||||
| Atrial fibrillation, n (%) | 181 (35.3) | 75 (29.6) | 44 (33.3) | 62 (48.4) | 0.001 |
| Hypertension, n (%) | 333 (64.9) | 169 (66.8) | 88 (66.7) | 76 (59.4) | 0.317 |
| Dyslipidemia, n (%) | 333 (64.9) | 173 (68.4) | 88 (66.7) | 72 (56.3) | 0.057 |
| Diabetes, n (%) | 186 (36.3) | 96 (37.9) | 57 (43.2) | 33 (25.8) | 0.057 |
| Chronic kidney disease, n (%) | 338 (65.9) | 162 (64.0) | 86 (65.2) | 90 (70.3) | 0.464 |
| Anemia, n (%) | 236 (46.0) | 108 (42.7) | 63 (47.7) | 65 (50.8) | 0.293 |
| Medications | |||||
| β‐blocker, n (%) | 351 (68.4) | 178 (70.4) | 85 (64.4) | 88 (68.8) | 0.488 |
| ACE‐I/ARB/ARNI, n (%) | 320 (62.4) | 166 (65.6) | 74 (56.1) | 80 (62.5) | 0.185 |
| ACE‐I, n (%) | 203 (39.6) | 103 (40.7) | 48 (36.4) | 52 (40.6) | 0.682 |
| ARB, n (%) | 138 (26.9) | 75 (29.6) | 28 (21.2) | 35 (27.3) | 0.207 |
| ARNI, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ‐ |
| Mineralocorticoid receptor antagonist, n (%) | 181 (35.3) | 70 (27.7) | 45 (34.1) | 66 (51.6) | <0.001 |
| Sodium glucose cotransporter 2 inhibitor, n (%) | 35 (6.8) | 17 (6.7) | 11 (8.3) | 7 (5.5) | 0.655 |
| Diuretic, n (%) | 333 (64.9) | 146 (57.7) | 84 (63.6) | 103 (80.5) | 0.001 |
Data are presented as mean±SD, median (interquartile range), or number (percentage). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; and HVSI, hepatic venous stasis index.
A P value indicates statistically significance in comparison across all groups.
Regarding laboratory data (Table 2), levels of total bilirubin, lactate dehydrogenase, alkaline phosphatase, 7S domain of collagen type IV, and BNP became higher, and levels of albumin, cholinesterase, and white blood cell count became lower with increasing HVSI. However, aspartate aminotransferase, alanine aminotransferase, C‐reactive protein, blood urea nitrogen, creatinine, sodium, and hemoglobin did not differ among the groups. Regarding the parameters of echocardiography, there was a significant increase in left atrial volume, inferior vena cava diameter, tricuspid regurgitation pressure gradient, and severity of tricuspid regurgitation, and a significant decrease in tricuspid annular plane systolic excursion with increasing HVSI. However, LVEF did not differ among the groups. Cardiopulmonary hemodynamics evaluated by RHC worsened with increasing HVSI. There was a gradual increase in mean RAP, mean pulmonary artery pressure, pulmonary artery wedge pressure, and PVR with increasing HVSI, whereas there was no significant change in cardiac index. Regarding abdominal ultrasonography, severity of HV waveform groups, RVSI, IRVF patterns, SWE, and SWD became higher with increasing HVSI. However, aorta preceliac peak systolic velocity and aorta preceliac velocity time integral did not differ among the groups. These results suggest that elevated HVSI indicates mainly hepatic congestion and right‐sided overload rather than liver hypoperfusion.
Table 2.
Laboratory Data of the Study Population
| Total (n=513) | HVSI=0 (HVSI=0, n=253) | Low HVSI (HVSI 0.01–0.20, n=32) | High HVSI (HVSI>0.20, n=128) | P value* | |
|---|---|---|---|---|---|
| Laboratory data | |||||
| Total protein, g/dL | 6.8±0.7 | 6.9±0.8 | 6.8±0.6 | 6.7±0.7 | 0.377 |
| Albumin, g/dL | 3.8 (3.4–4.2) | 3.9 (3.5–4.3) | 3.8 (3.5–4.1) | 3.7 (3.3–4.1) | 0.003 |
| Total bilirubin, mg/dL | 0.8 (0.6–1.0) | 0.7 (0.6–1.0) | 0.7 (0.5–1.0) | 0.9 (0.7–1.2) | <0.001 |
| Direct bilirubin, mg/dL | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.2) | <0.001 |
| Aspartate aminotransferase, U/L | 23.0 (18.0–29.0) | 23.0 (18.0–28.0) | 22.0 (19.0–29.0) | 23.5 (19.0–30.0) | 0.321 |
| Alanine aminotransferase, U/L | 18.0 (12.0–27.0) | 19.0 (13.0–29.8) | 16.0 (12.0–24.0) | 17.0 (13.3–26.0) | 0.094 |
| Lactate dehydrogenase, U/L | 217.0 (188.0–260.0) | 213.5 (182.0–250.8) | 222.0 (186.0–266.0) | 224.0 (200.0–267.8) | 0.039 |
| Alkaline phosphatase, U/L | 239.0 (188.0–295.0) | 231.0 (183.5–276.5) | 239.0 (185.5–303.0) | 262.0 (199.0–341.0) | 0.025 |
| Gamma‐glutamyl transferase, U/L | 35.0 (20.0–70.0) | 32.0 (19.0–62.0) | 30.5 (18.0–59.8) | 50.0 (27.0–92.0) | <0.001 |
| Cholinesterase, U/L | 250.0 (197.5–306.0) | 266.0 (214.0–324.0) | 240.5 (188.0–289.0) | 234.0 (173.0–282.0) | <0.001 |
| 7S domain of collagen type IV, ng/mL | 5.5 (4.5–6.9) | 5.2 (4.3–6.5) | 5.2 (4.5–6.4) | 6.7 (5.5–8.5) | <0.001 |
| C‐reactive protein, mg/dL | 0.24 (0.09–0.98) | 0.22 (0.08–0.86) | 0.22 (0.08–1.19) | 0.33 (0.10–1.22) | 0.373 |
| B‐type natriuretic peptide, pg/mL | 183.4 (78.7–374.7) | 129.1 (61.3–280.9) | 217.8 (111.8–420.8) | 223.1 (117.9–494.8) | <0.001 |
| Blood urea nitrogen, mg/dL | 19.0 (15.0–25.8) | 19.0 (15.0–25.0) | 20.0 (16.0–26.0) | 20.0 (15.0–25.8) | 0.740 |
| Creatinine, mg/dL | 1.00 (0.82–1.21) | 0.97 (0.82–1.19) | 0.99 (0.79–1.24) | 1.04 (0.83–1.21) | 0.684 |
| Estimated glomerular filtration rate, mL/min per 1.73 cm2 | 52.0 (40.3–64.0) | 53.0 (41.0–65.0) | 52.0 (37.0–63.0) | 50.0 (39.3–62.0) | 0.392 |
| Sodium, mEq/L | 140.0 (138.0–141.0) | 140.0 (138.0–141.0) | 140.0 (139.0–141.0) | 140.0 (138.0–142.0) | 0.231 |
| White blood cell, 103/μL | 5.5 (4.6–6.7) | 5.7 (4.8–6.9) | 5.4 (4.4–6.4) | 5.3 (4.5–6.6) | 0.047 |
| Hemoglobin, g/dL | 12.8 (11.2–14.4) | 12.9 (11.4–14.6) | 12.5 (10.7–14.1) | 12.6 (11.1–14.5) | 0.051 |
| Platelet, 103/μL | 193.0 (153.0–248.0) | 188.5 (152.0–243.0) | 191.0 (154.0–245.0) | 207.5 (154.3–257.3) | 0.566 |
| Echocardiography | |||||
| Left ventricle ejection fraction, % | 53.0 (34.7–62.0) | 55.0 (36.0–63.1) | 52.0 (35.0–62.0) | 50.0 (30.0–61.0) | 0.145 |
| Left ventricular outflow tract velocity‐time integral, cm | 16.0 (12.6–20.0) | 16.5 (13.2–19.9) | 16.3 (13.0–20.5) | 14.5 (11.0–19.4) | 0.015 |
| Left atrial volume, mL | 88.0 (66.0–119.7) | 82.0 (58.5–105.5) | 86.0 (67.0–117.2) | 109.5 (79.3–148.0) | <0.001 |
| Early trans mitral flow velocity to mitral annular velocity ratio | 13.8 (9.7–19.1) | 12.6 (9.1–16.6) | 15.4 (10.9–20.1) | 14.4 (10.8–21.6) | 0.001 |
| Right atrial end systolic area, cm2 | 18.6 (14.0–24.0) | 18.0 (14.0–23.0) | 15.0 (12.7–23.0) | 21.0 (17.1–27.5) | <0.001 |
| Right ventricle area diastole, cm2 | 19.4 (14.8–25.2) | 19.4 (15.4–26.0) | 15.6 (12.0–26.2) | 20.6 (16.8–24.7) | 0.073 |
| Right ventricle area systole, cm2 | 11.2 (9.0–16.8) | 11.9 (9.5–17.6) | 9.2 (7.3–13.8) | 13.5 (10.5–17.9) | 0.023 |
| Right ventricular fractional area change, % | 38.0 (32.0–44.0) | 38.0 (32.0–44.0) | 38.5 (31.6–46.0) | 32.0 (23.5–39.0) | <0.001 |
| Inferior vena cava diameter, mm | 15.0 (13.0–18.1) | 14.0 (12.0–17.0) | 15.0 (12.3–18.0) | 18.0 (14.7–21.0) | <0.001 |
| Tricuspid regurgitation pressure gradient, mm Hg | 24.0 (19.0–32.0) | 23.0 (18.8–31.0) | 24.0 (19.0–31.0) | 28.0 (20.5–36.5) | 0.005 |
| Tricuspid annular plane systolic excursion, mm | 17.2 (14.4–20.0) | 17.6 (14.8–20.3) | 17.2 (14.7–20.2) | 16.1 (12.1–19.5) | 0.009 |
| Tissue Doppler‐derived tricuspid lateral annular systolic velocity (tricuspid valve S′), cm/s | 9.8 (7.9–11.3) | 9.9 (8.0–11.3) | 10.2 (8.7–11.5) | 9.0 (7.5–10.5) | 0.081 |
| Tricuspid insufficiency, n (%) | |||||
| None‐trivial/mild/moderate/severe | 345 (67.3)/105 (20.5)/49 (9.6)/14 (2.7) | 176 (69.6)/52 (20.6)/24 (9.5)/1 (0.4) | 95 (72.0)/25 (18.9)/11 (8.3)/1 (0.8) | 74 (57.8)/28 (21.9)/14 (10.9)/12 (9.4) | <0.001 |
| Right heart catheterization (n=310) | |||||
| Mean right atrium pressure, mm Hg | 6.0 (4.0–9.3) | 6.0 (3.0–8.0) | 6.0 (4.0–9.0) | 8.0 (5.0–11.0) | <0.001 |
| Mean pulmonary artery pressure, mm Hg | 22.0 (17.0–31.0) | 20.0 (15.0–27.0) | 21.0 (17.0–30.0) | 25.0 (19.8–35.3) | <0.001 |
| Mean pulmonary artery wedge pressure, mm Hg | 13.0 (9.0–19.0) | 12.0 (7.0–17.0) | 14.0 (9.0–21.0) | 16.0 (12.0–21.0) | <0.001 |
| Cardiac index, L/min per m2 | 2.4 (2.0–2.8) | 2.4 (2.1–2.9) | 2.4 (2.1–2.8) | 2.2 (2.0–2.6) | 0.112 |
| Pulmonary vascular resistance (WoodU) | 2.0 (1.3–3.0) | 1.9 (1.3–2.8) | 1.9 (1.3–2.8) | 2.5 (1.8–4.6) | 0.005 |
| Abdominal ultrasonography | |||||
| Aorta preceliac peak systolic velocity, cm/s | 60.4 (48.3–73.8) | 59.7 (48.9–74.5) | 60.7 (46.5–73.7) | 60.9 (48.7–73.7) | 0.804 |
| Aorta preceliac velocity time integral, cm | 19.2 (15.5–23.4) | 19.2 (16.0–23.6) | 19.8 (15.6–23.4) | 18.3 (14.5–23.3) | 0.354 |
| Hepatic vein waveform groups | |||||
| C/U/R group, n (%) | 256 (49.9)/91 (17.7)/166 (32.4) | 239 (94.5)/14 (5.5)/0 (0) | 7 (5.3)/64 (48.5)/61 (46.2) | 10 (7.8)/13 (10.2)/105 (82.0) | <0.001 |
| Renal venous stasis index | 0.09 (0.00–0.23) | 0.00 (0.00–0.00) | 0.00 (0.08–0.13) | 0.24 (0.11–0.33) | <0.001 |
| Intrarenal venous flow patterns, n (%) | |||||
| Continuous/biphasic/monophasic pattern/others | 339 (67.9)/97 (19.4)/61 (12.2)/2 (0.4) | 210 (86.1)/20 (8.2)/14 (5.7)/0 (0.0) | 85 (65.4)/29 (22.3)/16 (12.3)/0 (0.0) | 44 (35.2)/48 (38.4)/31 (24.8)/2 (1.6) | <0.001 |
| Shear wave elastography, m/s | 1.36 (1.24–1.50) | 1.33 (1.22–1.46) | 1.33 (1.24–1.44) | 1.46 (1.31–1.66) | <0.001 |
| Shear wave dispersion, [m/s]/kHz | 10.0 (8.90–12.0) | 9.8 (8.8–11.0) | 9.8 (8.5–11.9) | 11.3 (9.5–14.3) | <0.001 |
Data are presented as mean±SD, or median (interquartile range), or number (percentage). HVSI indictes hepatic venous statis index.
A P value indicates statistically significance in comparison across all groups.
During the follow‐up period (median 517 days; range 1 to 1594 days, interquartile range 140.5 to 931.5 days), death from any cause occurred in 51 patients (30 cardiac deaths and 21 noncardiac deaths). We observed 87 cardiac events (29 cardiac deaths and 58 worsening HF). The 29 cardiac deaths included 26 deaths from HF and 3 deaths from ventricular fibrillation. There were 15 patients who experienced cardiac death and 4 patients who experienced noncardiac death after the first event of worsening HF. In the Kaplan–Meier analysis for cardiac event rate stratified by HVSI, the cumulative cardiac event rate significantly increased with increasing HVSI (Figure 3; log‐rank, P=0.002). In the Cox proportional hazard analysis (Table 3), due to the limited number of cardiac events (87 events) and to avoid overfitting, we selected the following factors: age, sex, body mass index, and confounding factors that were generally recognized as poor prognostic factors (ie, diabetes, CKD, AF, anemia, LVEF, and BNP). In the univariate Cox proportional hazard analysis, high HVSI was associated with high cardiac event rate (Table 3; low HVSI group versus HVSI 0 group: hazard ratio [HR], 1.739 [95% CI, 1.014–2.984], P=0.045, high HVSI group versus HVSI 0 group: HR, 2.335 [95% CI, 1.429–3.814], P<0.001). In the multivariable Cox proportional hazard analyses, we selected univariate factors with P<0.05 (ie, age, body mass index, diabetes, CKD, anemia, LVEF, and BNP). After adjusting for these confounding factors, high HVSI was an independent prognostic factor (Table 3; high HVSI group versus HVSI 0 group: HR, 2.037 [95% CI, 1.209–3.434], P=0.008). In the subgroup analysis regarding LVEF, there was no significant interaction between prognostic impact of HVSI and LVEF (P=0.340). In addition, in the Kaplan–Meier analysis for cardiac event rate stratified by HV waveform groups, the cumulative cardiac event rate was lowest in the C group (Figure 4; log‐rank, P=0.012). Furthermore, in the Kaplan–Meier analysis for cardiac event rate stratified by SWD, the cumulative cardiac event rate was significantly higher in the high SWD group than in the low SWD group (log‐rank, P=0.002). In addition, in the Kaplan–Meier analysis for cardiac event rate stratified by SWE, the cumulative cardiac event rate tended to be higher in the high SWE group than in the low SWE group (log‐rank, P=0.060).
Figure 3. Kaplan–Meier analysis for cardiac event rate stratified by HVSI.
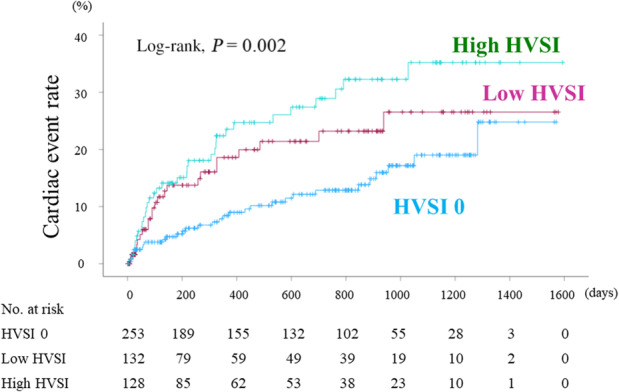
The cumulative cardiac event rate significantly increased with worsening HVSI (log‐rank, P=0.002). HVSI indicates hepatic venous stasis index.
Table 3.
Cox Proportional Hazard Model for Predicting Cardiac Events
| Cardiac events | Univariate | Multivariable* | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| Age | 1.024 | 1.006–1.042 | 0.008 | 1.018 | 0.996–1.040 | 0.109 |
| Sex (male=1) | 1.153 | 0.749–1.773 | 0.519 | |||
| Body mass index | 0.917 | 0.874–0.962 | <0.001 | 0.945 | 0.896–0.996 | 0.034 |
| Diabetes | 2.045 | 1.343–3.115 | <0.001 | 2.004 | 1.290–3.113 | 0.002 |
| Chronic kidney disease | 2.476 | 1.473–4.164 | <0.001 | 1.515 | 0.873–2.631 | 0.140 |
| Atrial fibrillation | 1.259 | 0.820–1.933 | 0.293 | |||
| Anemia | 1.780 | 1.164–2.721 | 0.008 | 1.368 | 0.865–2.165 | 0.180 |
| Left ventricle ejection fraction | 0.980 | 0.968–0.992 | <0.001 | 0.984 | 0.971–0.998 | 0.025 |
| B‐type natriuretic peptide | 1.001 | 1.001–1.002 | <0.001 | 1.001 | 1.001–1.001 | <0.001 |
| HVSI | ||||||
| Low HVSI vs HVSI 0 | 1.739 | 1.014–2.984 | 0.045 | 1.375 | 0.794–2.381 | 0.255 |
| High HVSI vs HVSI 0 | 2.335 | 1.429–3.814 | <0.001 | 2.037 | 1.209–3.434 | 0.008 |
HVSI indicates hepatic venous stasis index.
Adjusted for age, body mass index, diabetes, chronic kidney disease, anemia, left ventricle ejection fraction, and B‐type natriuretic peptide.
Figure 4. Kaplan–Meier analysis for cardiac event rate stratified by HV waveform groups.
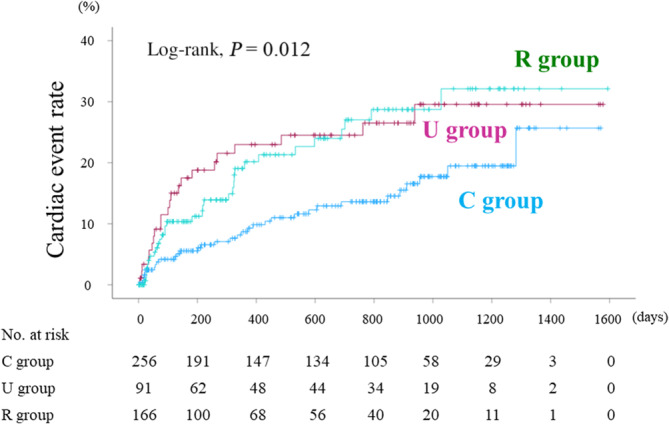
The cumulative cardiac event rate was lowest in the C group among the groups (log‐rank, P=0.012). HV indicates hepatic vein.
Subsequently, we used AHEAD score to test predictive ability of HVSI. In this study, the original AHEAD score demonstrated modest discrimination (c‐index=0.63 [95% CI, 0.57–0.70]). When HVSI was added to AHEAD score (Figure 5), the model discrimination was significantly improved (c‐index=0.67 [95% CI, 0.60–0.73], P=0.034).
Figure 5. Comparison of the ROC curves between the original AHEAD score and the modified AHEAD score (added HVSI).
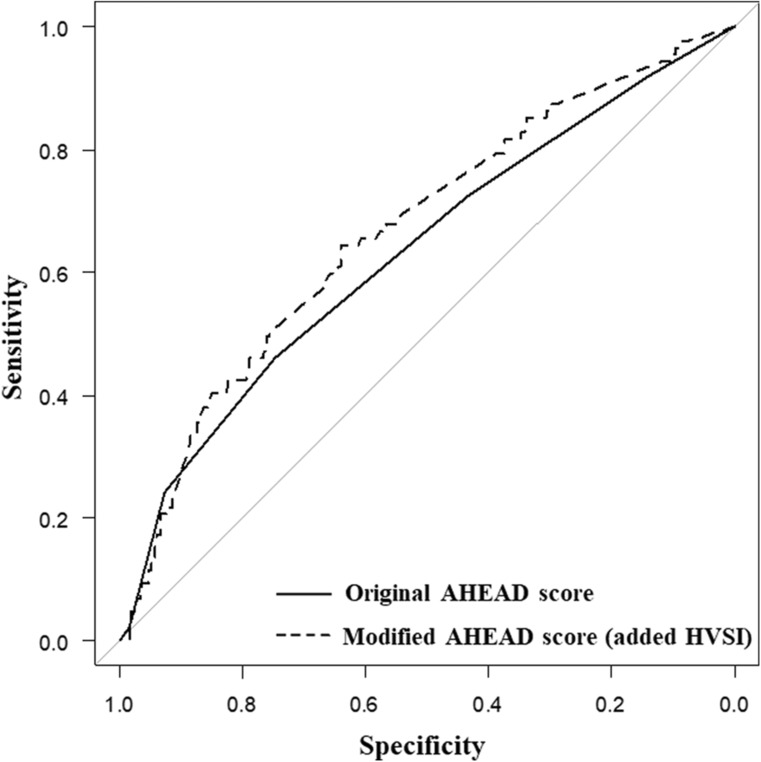
The AUCs for the original AHEAD score and the modified AHEAD score were 0.63 (95% CI, 0.57–0.70) and 0.67 (95% CI, 0.60–0.73), respectively. The AHEAD score is based on the following comorbidities: A–atrial fibrillation, H–hemoglobin levels of <12.0 g/dL in women and <13.0 g/dL in men (anemia), E–elderly (age >70 years), A–abnormal renal parameters (creatinine >130 μmol/L), D, diabetes. AUC indicates area under the curve; HVSI, hepatic venous stasis index; and ROC, receiver operating characteristic.
DISCUSSION
In the present study, we investigated the associations of HVSI assessed by abdominal ultrasonography with laboratory tests, echocardiography, and RHC, as well as its prognostic impact in patients with HF. The present study is, to the best of our knowledge, the first to report that HVSI is a quantitative marker of hepatic congestion and associated with liver dysfunction, right‐sided overload, and higher cardiac events in patients with HF.
Cardio‐Hepatic Interaction
As it receives up to 25% of total cardiac output, the liver is very sensitive to hemodynamic modulation and heart dysfunction. 7 Cardio‐hepatic interaction such as congestive hepatopathy (passive congestion secondary to increased CVP) and hypoxic hepatitis (reduced arterial perfusion) in patients with HF has been reported. 1 , 7 , 8 , 10 , 11 , 12 , 13 Passive congestion prevails in congestive hepatopathy secondary to chronic HF, whereas arterial hypoperfusion predominates in acute HF leading to hypoxic hepatitis. However, these forward and backward factors often coexist and potentiate each other. 12 The starting point of congestive hepatopathy is an increase in the CVP secondary to right HF with a high level of filling pressure. Due to the absence of valves in HV, increased CVP affects the sinusoidal bed causing centrilobular congestion, sinusoidal dilation, and perivenular fibrosis. 1 , 7 , 10 , 13 , 52 , 53 , 54 Sinusoidal dilation is positively correlated with the degree of elevation of RAP and explains the hepatomegaly frequently observed in patients with congestive hepatopathies. 55 The major damage occurs in the Rappaport acinus, which surrounds the venae centralis hepatis. 12 , 13 Centrilobular necrosis can extend to peripheral areas if HF persists and worsens, and causes deposition and spread of connective tissue bridging one venae centralis hepatis to the other, ultimately leading to cirrhosis. 7 , 56 Hypoxic hepatitis is a clinical and histological syndrome leading to the reduction of hepatic arterial perfusion due to acute fall in cardiac output. 52 , 54 Reduced hepatic arterial perfusion can cause hypoxic hepatopathy, and hypoxia can cause centrilobular necrosis and sinusoidal damage in the liver, leading to elevation of transaminase. 54 Concerning heart disease that be caused by a liver disease, cirrhotic cardiomyopathy is present. 57 It is a syndrome characterized by systolic dysfunction, impaired diastolic relaxation, prolonged isovolumetric relaxation time, and electrophysiological disturbances such as prolonged QTc interval. 58
Hepatic Vein Waveforms
HV waveforms are reported to be useful for the assessment of increased CVP and prognosis of cardiac event rate in patients with HF. 22 The downward Doppler signal indicates the venous return flow (antegrade flow), the upward Doppler signal indicates the reversed blood flow to the liver from the heart (retrograde flow), and no signal indicates stasis flow. HV waveform is primarily composed of 2 distinct forward systolic and diastolic waves (S wave and D wave) during ventricular systole and early diastole, and 2 small reverse flow during late ventricular systole and atrial systole (V wave and A wave). 43 , 44 , 46 , 59 , 60 However, the V wave is transitional, which may be antegrade, retrograde, or neutral. 61 Along with the increasing of RAP, the flow reverses to the vena cava in the right atrial systole. In the HV waveform, it shows as a retrograde A wave (Figure 2E). After the end systole, as the ventricular contraction intensity decreases and the closed tricuspid valve begins to return to its original resting position, the right atrium fills, venous return velocity decreases, and temporary equilibrium is reached, making V waves. 43 Therefore, the V wave corresponds to right atrial overfilling. In patients with high right ventricular end diastolic pressure, the right atrium must contract with greater force to drive blood into the right ventricle. This results in increased RAP, leading to an augmentation of the retrograde blood flow into the vena cava (Figure 2F). If the A, S, and V waves are all retrograde, they may fuse into a single retrograde wave and become biphasic waveforms, alternating with the D wave (Figure 2G). 44
With regard to the HV waveforms, it has been suggested that the significance of HV waveforms differ depending on the disease. 43 , 62 In patients with liver cirrhosis, the continuous HV waveform (namely Figure 2A with HVSI=0 in this study) is mainly caused by inflammatory or fibrotic changes, intrahepatic fat deposition, and changes in the compliance of the venous wall, suggesting the presence of severe liver fibrosis. 63 , 64 , 65 Because we excluded the patients with distinct liver disease (including liver cirrhosis), we were able to measure the change of HV waveform due to not liver disease but HF, and observe associations between HVSI and RAP. Therefore, the results of this study may have been in contrast to those of previous studies on HV waveform in patients with liver cirrhosis.
The Utility of HVSI
We here report the utility of HVSI for estimating hepatic congestion, liver dysfunction, RAP, and prognosis. First, the classification of HV waveform groups has a weakness; it may be difficult to classify some patterns. HVSI can complement the weaknesses by quantifying HV waveforms. We consider that quantifying HVSI can detect hepatic congestion more sensitively than classification of HV waveform groups. Second, the measurement of SWE and SWD requires specific abdominal ultrasonographic equipment and is not easy for cardiologists. HVSI needs neither special equipment nor complex measurements, cardiologists can measure HVSI through training a few times. Third, according to the past study, RVSI and IRVF flow patterns are useful as a prognostic indicator for HF. 17 , 18 , 21 , 23 , 24 However, they differ in that HVSI reflects hepatic congestion and RVSI and IRVF flow patterns reflect renal congestion. In terms of assessing organ congestion, there is value in measuring both HVSI and RVSI or IRVF flow patterns. RVSI and IRVF flow patterns have to detect both intrarenal arterial flow and intrarenal venous flow at the same time, which is often difficult. HVSI has only to detect HV waveform. The size of hepatic vein is 6 to 7 mm and enough to be easily measured. In addition, HV is closer in anatomic proximity to the right heart than intrarenal vessels. In the present study, the measurement of HVSI seemed to be superior to those of RVSI and IRVF patterns, in terms of ease of measurement and accuracy. As shown in Figure 4, regarding comparison of HVSI with HV waveform groups for predicting prognosis, Kaplan–Meier analysis of HV waveform groups showed overlap and inversion between the U group and R group. Our data suggest that the HVSI can be superior to HV waveform groups. Patients with HF and high HVSI despite treatment can have remaining hepatic congestion and lead to adverse prognosis. Thus, it appears that cardioprotective agents or decreasing central venous pressure by diuretic agents may be useful to decrease HVSI and improve prognosis in these patients.
Study Limitations
There are some limitations in the present study. First, the study might be underpowered because it was a single center prospective cohort study with a relatively small sample size and a short follow‐up period. Second, although documented liver disease was excluded in this study, we could not fully exclude other liver diseases that may have affected the HVSI. Third, we used only data during hospitalization and did not consider changes in medical parameters or treatments after discharge. Future studies need to assess alterations of HVSI in response to hemodynamic changes because the hemodynamics of patients with HF change dynamically. Fourth, we could not perform abdominal ultrasonography in all hospitalized patients. In addition, there might be a potential selection bias since the attending physicians made decisions to perform RHC. Therefore, the present results need to be viewed as preliminary, and further studies with a larger number of patients are needed.
CONCLUSIONS
HVSI assessed by abdominal ultrasonography reflects hepatic congestion and right‐sided HF, and is associated with adverse prognosis in patients with HF.
Sources of Funding
This study was supported in part by a grant‐in‐aid for Scientific Research (20K07828 and 20K16529) from the Japan Society for the Promotion of Science.
Disclosures
None.
Acknowledgments
H.O. and Y. Sato: conceptualization, methodology, formal analysis, investigation, writing–original draft, and visualization. A.Y.: conceptualization, methodology, formal analysis, investigation, resources, data curation, writing–original draft, visualization, supervision, project administration, and funding acquisition. S.I., M.M., and Y.Y.: methodology, investigation, writing–original draft, and visualization. Y. Sugawara, Y.I., T.M., T.S., M.O., and A.K.: conceptualization, methodology, formal analysis, investigation, and writing–review and editing. Y.T.: conceptualization, methodology, formal analysis, investigation, writing–original draft, supervision, and project administration. All authors contributed to the article and approved the submitted version.
This article was sent to Sakima Smith, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 12.
References
- 1. Samsky MD, Patel CB, DeWald TA, Smith AD, Felker GM, Rogers JG, Hernandez AF. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol. 2013;61:2397–2405. doi: 10.1016/j.jacc.2013.03.042 [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, Aragam J, Bemjamin EJ, Larson MG. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham Study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11:1–11. doi: 10.1016/j.jcmg.2017.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.CIR.0000039105.49749.6F [DOI] [PubMed] [Google Scholar]
- 6. Okura Y, Ramadan MM, Ohno Y, Mitsuma W, Tanaka K, Ito M, Suzuki K, Tanabe N, Kodama M, Aizawa Y. Impending epidemic: future projection of heart failure in Japan to the year 2055. Circ J. 2008;72:489–491. doi: 10.1253/circj.72.489 [DOI] [PubMed] [Google Scholar]
- 7. Moller S, Bernardi M. Interactions of the heart and the liver. Eur Heart J. 2013;34:2804–2811. doi: 10.1093/eurheartj/eht246 [DOI] [PubMed] [Google Scholar]
- 8. van Deursen VM, Damman K, Hillege HL, van Beek AP, van Veldhuisen DJ, Voors AA. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail. 2010;16:84–90. doi: 10.1016/j.cardfail.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 9. Mebazaa A. Congestion and cardiorenal syndromes. Contrib Nephrol. 2010;165:140–144. doi: 10.1159/000313752 [DOI] [PubMed] [Google Scholar]
- 10. Nikolaou M, Parissis J, Yilmaz MB, Seronde MF, Kivikko M, Laribi S, Paugram‐Burtz C, Cai D, Pohjanjousi P, Laterre PF, et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur Heart J. 2013;34:742–749. doi: 10.1093/eurheartj/ehs332 [DOI] [PubMed] [Google Scholar]
- 11. Jalal Z, Iriart X, De Ledinghen V, Barnetche T, Hiriart JB, Vergniol J, Foucher J, Thambo JB. Liver stiffness measurements for evaluation of central venous pressure in congenital heart diseases. Heart. 2015;101:1499–1504. doi: 10.1136/heartjnl-2014-307385 [DOI] [PubMed] [Google Scholar]
- 12. Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–1070. doi: 10.2169/internalmedicine.46.0059 [DOI] [PubMed] [Google Scholar]
- 13. Koehne de Gonzalez AK, Lefkowitch JH. Heart disease and the liver: pathologic evaluation. Gastroenterol Clin North Am. 2017;46:421–435. doi: 10.1016/j.gtc.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 14. Massari F, Scicchitano P, Iacoviello M, Passantino A, Guida P, Sanasi M, Piscopo A, Romito R, Valle R, Caldarola P, et al. Multiparametric approach to congestion for predicting long‐term survival in heart failure. J Cardiol. 2020;75:47–52. doi: 10.1016/j.jjcc.2019.05.017 [DOI] [PubMed] [Google Scholar]
- 15. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, Kenizou D, Maillier B, Nazeyrollas P, Roul G, et al. Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Fail. 2018;6:273–285. doi: 10.1016/j.jchf.2017.09.023 [DOI] [PubMed] [Google Scholar]
- 16. Pellicori P, Kaur K, Clark AL. Fluid management in patients with chronic heart failure. Card Fail Rev. 2015;1:90–95. doi: 10.15420/cfr.2015.1.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshihisa A, Watanabe K, Sato Y, Ishibashi S, Matsuda M, Yamadera Y, Ichijo Y, Yokokawa T, Misaka T, Oikawa M, et al. Intrarenal Doppler ultrasonography reflects hemodynamics and predicts prognosis in patients with heart failure. Sci Rep. 2020;10:22257. doi: 10.1038/s41598-020-79351-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iida N, Seo Y, Sai S, Machino‐Ohtsuka T, Yamamoto M, Ishizu T, Kawakami Y, Aonuma K. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4:674–682. doi: 10.1016/j.jchf.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 19. Yoshihisa A, Ishibashi S, Matsuda M, Yamadera Y, Ichijo Y, Sato Y, Yokokawa T, Misaka T, Oikawa M, Kobayashi A, et al. Clinical implications of hepatic hemodynamic evaluation by abdominal ultrasonographic imaging in patients with heart failure. J Am Heart Assoc. 2020;9:e016689. doi: 10.1161/JAHA.120.016689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohara H, Yoshihisa A, Ishibashi S, Matsuda M, Yamadera Y, Sugawara Y, Ichijo Y, Hotsuki Y, Watanabe K, Anzai F, et al. Shear wave dispersion predicts liver fibrosis and adverse outcomes in patients with heart failure. J Clin Med. 2020;9:3953. doi: 10.3390/jcm9123953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohara H, Yoshihisa A, Horikoshi Y, Ishibashi S, Matsuda M, Yamadera Y, Sugawara Y, Ichijo Y, Hotsuki Y, Watanabe K, et al. Renal venous stasis index reflects renal congestion and predicts adverse outcomes in patients with heart failure. Front Cardiovasc Med. 2022;9:772466. doi: 10.3389/fcvm.2022.772466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sugawara Y, Yoshihisa A, Ishibashi S, Matsuda M, Yamadera Y, Ohara H, Ichijo Y, Watanabe K, Hotsuki Y, Anzai F, et al. Liver congestion assessed by hepatic vein waveforms in patients with heart failure. CJC Open. 2021;3:778–786. doi: 10.1016/j.cjco.2021.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto M, Seo Y, Iida N, Ishizu T, Yamada Y, Nakatsukasa T, Nakagawa D, Kawamatsu N, Sato K, Ohtsuka TM, et al. Prognostic impact of changes in intrarenal venous flow pattern in patients with heart failure. J Card Fail. 2021;27:20–28. doi: 10.1016/j.cardfail.2020.06.016 [DOI] [PubMed] [Google Scholar]
- 24. Seo Y, Iida N, Yamamoto M, Ishizu T, Ieda M, Ohte N. Doppler‐derived intrarenal venous flow mirrors right‐sided heart hemodynamics in patients with cardiovascular disease. Circ J. 2020;84:1552–1559. doi: 10.1253/circj.CJ-20-0332 [DOI] [PubMed] [Google Scholar]
- 25. Taniguchi T, Ohtani T, Kioka H, Tsukamoto Y, Onishi T, Nakamoto K, Katsimichas T, Sengoku K, Chimura M, Hashimoto H, et al. Liver stiffness reflecting right‐sided filling pressure can predict adverse outcomes in patients with heart failure. JACC Cardiovasc Imaging. 2019;12:955–964. doi: 10.1016/j.jcmg.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 26. Taniguchi T, Sakata Y, Ohtani T, Mizote I, Takeda Y, Asano Y, Masuda M, Minamiguchi H, Kanzaki M, Ichibori Y, et al. Usefulness of transient elastography for noninvasive and reliable estimation of right‐sided filling pressure in heart failure. Am J Cardiol. 2014;113:552–558. doi: 10.1016/j.amjcard.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 27. Colli A, Pozzoni P, Berzuini A, Gerosa A, Canovi C, Molteni EE, Barbarini M, Bonino F, Prati D. Decompensated chronic heart failure: increased liver stiffness measured by means of transient elastography. Radiology. 2010;257:872–878. doi: 10.1148/radiol.10100013 [DOI] [PubMed] [Google Scholar]
- 28. Soloveva A, Kobalava Z, Fudim M, Ambrosy AP, Villevalde S, Bayarsaikhan M, Garmash I, Naumenko M. Relationship of liver stiffness with congestion in patients presenting with acute decompensated heart failure. J Card Fail. 2019;25:176–187. doi: 10.1016/j.cardfail.2019.01.020 [DOI] [PubMed] [Google Scholar]
- 29. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 30. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri JB, Celutkiene J, Chioncel O, Clelamd JGF, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 31. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure‐ digest version. Circ J. 2019;83:2084–2184. doi: 10.1253/circj.CJ-19-0342 [DOI] [PubMed] [Google Scholar]
- 32. Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, Makaya M, Murohara T, Node K, Saito Y, et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. Circ J. 2021;85:2252–2291. doi: 10.1253/circj.CJ-21-0431 [DOI] [PubMed] [Google Scholar]
- 33. Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, Kinhart A, Widimsky P, Miklik R, Zeman K, et al. AHEAD score—long‐term risk classification in acute heart failure. Int J Cardiol. 2016;202:21–26. doi: 10.1016/j.ijcard.2015.08.187 [DOI] [PubMed] [Google Scholar]
- 34. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 35. Authors/Task Force Members, Guidelines ESC Committee for Practice Guidelines (CPG), ESC National Cardiac Societies . ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 36. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007 [DOI] [PubMed] [Google Scholar]
- 38. Miura S, Yoshihisa A, Takiguchi M, Shimizu T, Nakamura Y, Yamauchi H, Iwaya S, Owada T, Miyata M, Abe S, et al. Association of hypocalcemia with mortality in hospitalized patients with heart failure and chronic kidney disease. J Card Fail. 2015;21:621–627. doi: 10.1016/j.cardfail.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 39. Sato Y, Yoshihisa A, Oikawa M, Nagai T, Yoshikawa T, Saito Y, Yamamoto K, Takeishi Y, Anzai T. Prognostic impact of worsening renal function in hospitalized heart failure patients with preserved ejection fraction: a report from the JASPER registry. J Card Fail. 2019;25:631–642. doi: 10.1016/j.cardfail.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 40. Sirbu O, Floria M, Dascalita P, Stoica A, Adascalitei P, Sorodoc V, Sorodoc L. Anemia in heart failure—from guidelines to controversies and challenges. Anatol J Cardiol. 2018;20:52–59. doi: 10.14744/AnatolJCardiol.2018.08634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Babitt JL, Eisenga MF, Haase VH, Kshirsagar AV, Levin A, Locatelli F, Malyszko J, Swinkels DW, Tarng DC, Cheung M, et al. Controversies in optimal anemia management: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) conference. Kidney Int. 2021;99:1280–1295. doi: 10.1016/j.kint.2021.03.020 [DOI] [PubMed] [Google Scholar]
- 42. Husain‐Syed F, Birk HW, Ronco C, Schormann T, Tello K, Richter MJ, Wilhelm J, Sommer N, Steyerberg E, Bauer P, et al. Doppler‐derived renal venous stasis index in the prognosis of right heart failure. J Am Heart Assoc. 2019;8:e013584. doi: 10.1161/JAHA.119.013584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scheinfeld MH, Bilali A, Koenigsberg M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics. 2009;29:2081–2098. doi: 10.1148/rg.297095715 [DOI] [PubMed] [Google Scholar]
- 44. Sun DD, Hou CJ, Yuan LJ, Duan YY, Hou Y, Zhou FP. Hemodynamic changes of the middle hepatic vein in patients with pulmonary hypertension using echocardiography. PLoS One. 2015;10:e0121408. doi: 10.1371/journal.pone.0121408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nakatsuka T, Soroida Y, Nakagawa H, Shindo T, Sato M, Soma K, Nakagomi R, Kobayashi T, Endo M, Hikita H, et al. Identification of liver fibrosis using the hepatic vein waveform in patients with Fontan circulation. Hepatol Res. 2019;49:304–313. doi: 10.1111/hepr.13248 [DOI] [PubMed] [Google Scholar]
- 46. McNaughton DA, Abu‐Yousef MM. Doppler US of the liver made simple. Radiographics. 2011;31:161–188. doi: 10.1148/rg.311105093 [DOI] [PubMed] [Google Scholar]
- 47. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyakin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 48. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–688. doi: 10.1016/j.echo.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 49. Zaidi A, Oxborough D, Augustine DX, Bedair R, Harkness A, Rana B, Robinson S, Badano LP. Echocardiographic assessment of the tricuspid and pulmonary valves: a practical guideline from the British Society of Echocardiography. Echo Res Pract. 2020;7:G95–G122. doi: 10.1530/ERP-20-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. 2013;305:H1373–H1381. doi: 10.1152/ajpheart.00157.2013 [DOI] [PubMed] [Google Scholar]
- 51. Krishnan A, Markham R, Savage M, Wong YW, Walters D. Right heart catheterisation: how to do it. Heart Lung Circ. 2019;28:e71–e78. doi: 10.1016/j.hlc.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 52. Xanthopoulos A, Starling RC, Kitai T, Triposkiadis F. Heart failure and liver disease: cardiohepatic interactions. JACC Heart Fail. 2019;7:87–97. doi: 10.1016/j.jchf.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 53. Giallourakis CC, Rosenberg PM, Friedman LS. The liver in heart failure. Clin Liver Dis. 2002;6:947–967. doi: 10.1016/S1089-3261(02)00056-9 [DOI] [PubMed] [Google Scholar]
- 54. El Hadi H, Di Vincenzo A, Vettor R, Rossato M. Relationship between heart disease and liver disease: a two‐way street. Cells. 2020;9:567. doi: 10.3390/cells9030567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sessa A, Allaire M, Lebray P, Medmoun M, Tiritilli A, Iaria P, Cadranel JF. From congestive hepatopathy to hepatocellular carcinoma, how can we improve patient management? JHEP Rep. 2021;3:100249. doi: 10.1016/j.jhepr.2021.100249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sherlock S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br Heart J. 1951;13:273–293. doi: 10.1136/hrt.13.3.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Voiosu AM, Daha IC, Voiosu TA, Mateescu BR, Dan GA, Baicus CR, Voiosu MR, Diculescu MM. Prevalence and impact on survival of hepatopulmonary syndrome and cirrhotic cardiomyopathy in a cohort of cirrhotic patients. Liver Int. 2015;35:2547–2555. doi: 10.1111/liv.12866 [DOI] [PubMed] [Google Scholar]
- 58. Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol. 2014;11:177–186. doi: 10.1038/nrgastro.2013.210 [DOI] [PubMed] [Google Scholar]
- 59. Teichgraber UK, Gebel M, Benter T, Manns MP. Duplex ultrasound characterization of hepatic vein blood flow in healthy probands. Ultraschall Med. 1997;18:267–271. doi: 10.1055/s-2007-1000440 [DOI] [PubMed] [Google Scholar]
- 60. Lim AK, Patel N, Eckersley RJ, Kuo YT, Goldin RD, Thomas HC, Cosgrove DO, Taylor‐Robonson SD, Blomley MJK. Can Doppler sonography grade the severity of hepatitis C‐related liver disease? AJR Am J Roentgenol. 2005;184:1848–1853. doi: 10.2214/ajr.184.6.01841848 [DOI] [PubMed] [Google Scholar]
- 61. Pedersen JF, Dakhil AZ, Jensen DB, Sondergaard B, Bytzer P. Abnormal hepatic vein doppler waveform in patients without liver disease. Br J Radiol. 2005;78:242–244. doi: 10.1259/bjr/15227254 [DOI] [PubMed] [Google Scholar]
- 62. Fadel BM, Alassas K, Husain A, Dahdouh Z, Di Salvo G. Spectral Doppler of the hepatic veins in noncardiac diseases: what the echocardiographer should know. Echocardiography. 2015;32:1424–1427. doi: 10.1111/echo.12994 [DOI] [PubMed] [Google Scholar]
- 63. Bolondi L, Li Bassi S, Gaiani S, Zironi G, Benzi G, Santi V, Barbara L. Liver cirrhosis: changes of Doppler waveform of hepatic veins. Radiology. 1991;178:513–516. doi: 10.1148/radiology.178.2.1987617 [DOI] [PubMed] [Google Scholar]
- 64. Dietrich CF, Lee JH, Gottschalk R, Herrmann G, Sarrazin C, Caspary WF, Zeuzem S. Hepatic and portal vein flow pattern in correlation with intrahepatic fat deposition and liver histology in patients with chronic hepatitis C. AJR Am J Roentgenol. 1998;171:437–443. doi: 10.2214/ajr.171.2.9694471 [DOI] [PubMed] [Google Scholar]
- 65. Karabulut N, Kazil S, Yagci B, Sabir N. Doppler waveform of the hepatic veins in an obese population. Eur Radiol. 2004;14:2268–2272. doi: 10.1007/s00330-004-2423-0 [DOI] [PubMed] [Google Scholar]


