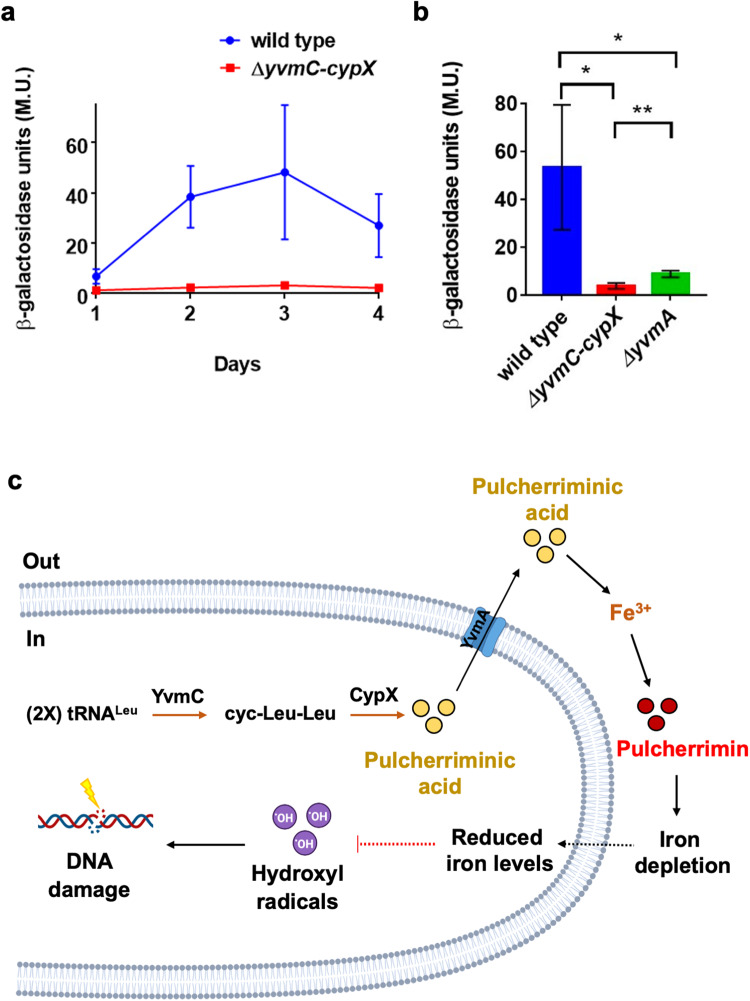
Fig. 8. Pulcherrimin production could reduce iron toxicity by lowering iron levels.
a The promoter activity for the bacillibactin biosynthetic operon was compared between the pulcherrimin mutant (LA148) and the wild type (YQ141) by using the transcriptional reporter PdhbA-lacZ. β-galactosidase assays were performed using pellicle biofilms of the two reporter strains collected every 24 hours over the course of 4 days. b A mutant for the pulcherrimin transporter (ΔyvmA) was also tested for the activation of the PdhbA-lacZ reporter (LA262). Pellicle biofilms of the three reporter strains were harvested after 72 h, and β-galactosidase assays were performed accordingly. Results show a significant decrease in promoter activation in ΔyvmA compared with the wild type (p = 0.0417, *), but higher activation compared with the pulcherrimin mutant (p = 0.0097, **). c A working model for the function of pulcherrimin during B. subtilis biofilm development as an iron-buffering molecule. This model shows that pulcherriminic acid molecules are produced intracellularly via two enzymatic reactions carried out by YvmC and CypX, respectively, using tRNA-charged leucine as the substrate. Pulcherriminic acid molecules are exported, via a dedicated transporter YvmA, to the extracellular environment where it binds to free ferric iron ions and turns into the reddish pigment pulcherrimin. Because iron-bound pulcherrimin is insoluble, iron depletion occurs extracellularly upon large quantities of secreted pulcherriminic acid molecules competitively chelating iron. This depletion likely results in lowered intracellular iron levels, which then leads to reduced Fenton reactions and less accumulation of DNA-damaging hydroxyl radicals. Thus, pulcherrimin production acts as an extracellular iron-buffering mechanism to reduce ROS production, consequently lowering oxidative stress and preventing DNA damage in B. subtilis. Model created with BioRender.com.