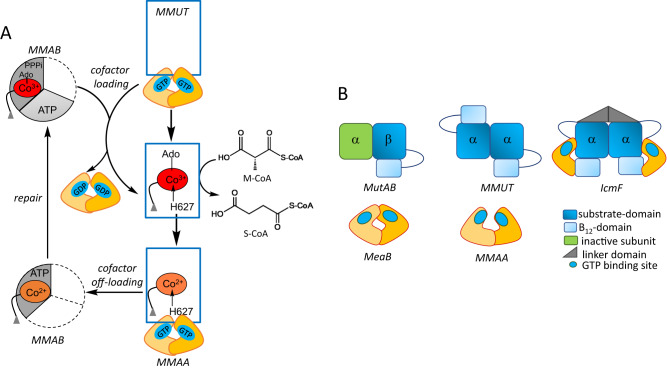
Fig. 1. Mitochondrial human B12 trafficking proteins and comparison in their organization with bacterial homologs.
A Model for cofactor loading and off-loading in the mitochondrial B12-trafficking pathway. After AdoCbl is loaded onto MMUT (blue) by MMAB (grey), MMUT catalyzes the isomerization of methymalonyl-CoA (M-CoA) to succinyl-CoA (S-CoA). The GAP function of MMUT enhances the GTPase activity of MMAA (yellow), which assists in the transfer of cofactor to/from MMUT. MMAB is an adenosyltransferase and converts cob(II)alamin to AdoCbl and loads it onto MMUT. B Cartoons showing the topological differences between bacterial MutAB, human MMUT, and IcmF.