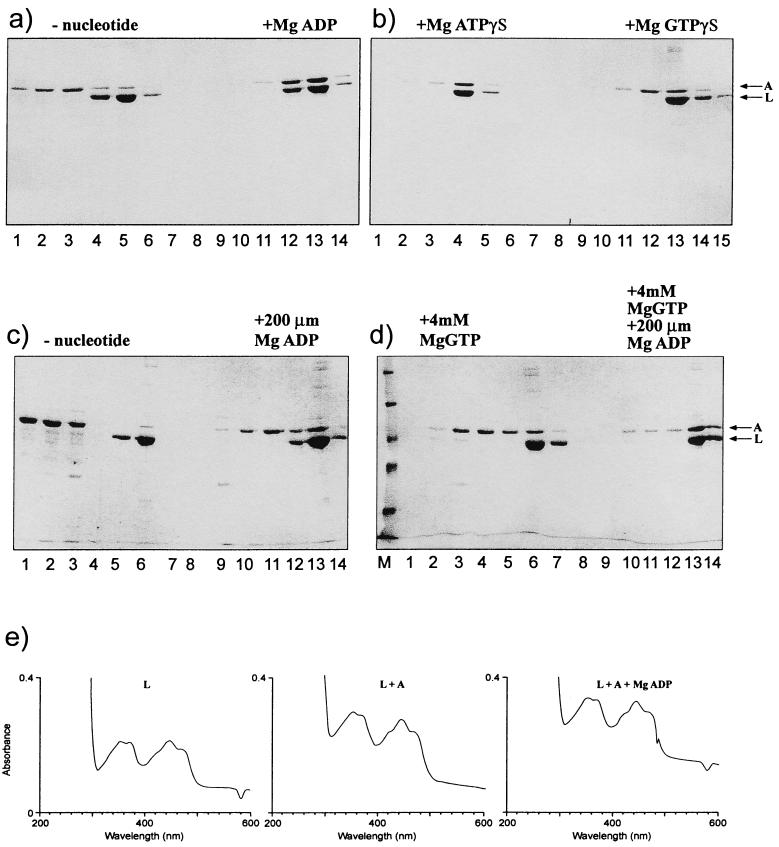
FIG. 1.
NIFL-NIFA complex formed in the presence of nucleotides. Complexes were formed and chromatographed as described in Materials and Methods. Electrophoresis was carried out on SDS-polyacrylamide gels (10% polyacrylamide). Full-length NIFL6his and NIFA were used at 2.5 μM, and nucleotides were used at 1 mM. (a) Complex formation with 1 mM MgADP. Lanes 1 to 3 and 9 to 11, wash fractions; lanes 4 to 6 and 12 to 14, fractions containing bound protein which eluted with 0.5 M imidazole. (b) Complex formation with 1 mM MgATPγS or GTPγS. Lanes 1 to 3 and 10 to 12, wash fractions; lanes 4 to 6 and 13 to 15, fractions containing protein obtained after elution. (C) Complex formation with 200 μM MgADP. Lanes 1 to 3 and 9 to 11, wash fractions; lanes 5 to 6 and 12 to 13, fractions containing protein obtained after elution. (D) Complex formation with 4 mM GTP in the presence and absence of 200 μM MgADP. Lanes 3 to 5 and 10 to 12, wash fractions; lanes 6 to 7 and 13 to 14, fractions containing protein obtained after elution. (E) Absorbance spectra of oxidized NIFL in the presence and absence of NIFA and 1 mM MgADP were measured in a Hewlett-Packard diode array spectrophotometer.