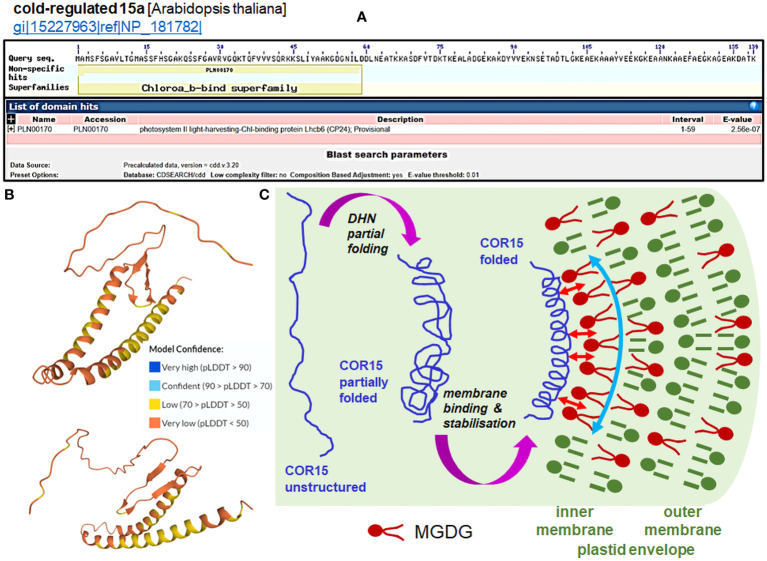
Figure 3.
Function of the COR15 proteins (data in panels A and B for COR15a only). (A) Prediction of the domain of the Arabidopsis COR15a protein (NP_181782.1; all data accessed from ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?seqinput=NP_181782.1). (B) Predicted 3D model of AtCOR15a (Q42512; CR15A_ARATH UniProt record) by AlphaFold (accessed from uniprot.org/uniprotkb/Q42512/entry#structure). Exemplary projections with a particularly low per-residue confidence score (pLDDT) through all protein residues indicate a lack of apparent structure in diverse protein regions of COR15. Enhancing the stabile folding of COR15 is generated by the membrane interaction (see panel (C). (C) Cell dehydration caused by freezing results in a closer apposition of intracellular membranes to each other and macromolecule crowding, which causes partial folding of the COR15 protein (first magenta arrow). Thalhammer and Hincha (2014) estimated that COR15 can occupy ca. 15% of the inner membrane of the surface of the plastid envelope. Plastid membranes contain monogalactosyl-diacylglycerol glycolipids (MGDG), which are unstable under low temperature. The COR15 protein interacts with the inner membrane of the plastid envelope enriched with MGDG (small red arrows), which improves the helical conformation of COR15 (second magenta arrow). Binding of COR15 to the MGDG-enriched plastid membrane occurs with the hydrophobic face of the amphipathic helix of COR15. This association results in enhanced folding, which exposes a larger hydrophobic face capable of interaction with membrane lipids. In addition, the helix may be stabilized on the membrane by hydrogen bonding with MGDG, which orients it parallel to the membrane surface. Overall, it provides stabilization of the membrane (curved vertical arrow) under freezing stress, thus preventing the formation of hexagonal phase II lipid domains. Such membrane stabilization maintains plastid integrity and functionality, as can be indicated by the electrolyte leakage test (panel (C) partly based on the concept from the data of Thalhammer and Hincha, 2014).