Figure 1.
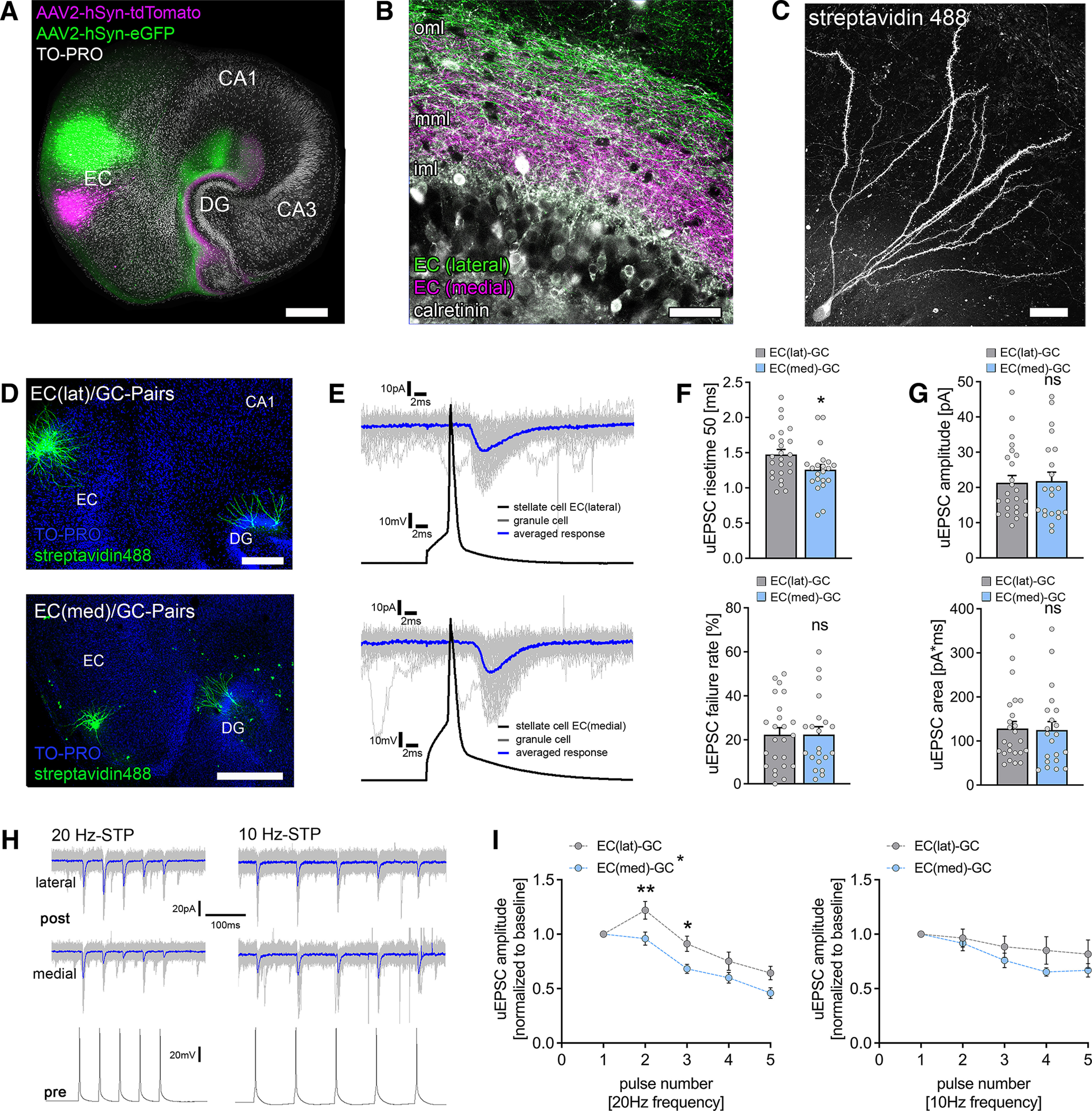
Functional characterization of laminar innervation of dentate granule cells in organotypic entorhino-hippocampal tissue cultures. A, Overview of a mouse organotypic entorhino-hippocampal tissue culture stained with TO-PRO nuclear stain (white) to visualize cytoarchitecture. The entorhino-hippocampal projection is visualized by a perforant path tracing using AAV-mediated expression of tdTomato (medial part) and eGFP (lateral part) in the entorhinal cortex. Scale bar, 300 µm. DG, Dentate gyrus. B, The dentate gyrus at higher magnification demonstrating its laminar innervation. eGFP-labeled axonal projections from the lateral part of the entorhinal cortex reach the outer molecular layer (oml), whereas tdTomato-labeled fibers project to the middle molecular layer (mml). The inner molecular layer (iml) that contains hilar mossy cell axons (visualized by calretinin immunostaining) is not innervated by entorhino-hippocampal projections. Scale bar, 50 µm. C, Post hoc streptavidin-stained dentate granule cell. Scale bar, 20 µm. D, Post hoc staining of paired recordings from layer 2 stellate cells in either the lateral (top) or the medial part (bottom) of the entorhinal cortex and dentate granule cells in the suprapyramidal blade of the dentate gyrus. TO-PRO nuclear stain was used to visualize cytoarchitecture. Scale bars: (top) 200 µm; (bottom) 300 µm. E, Action potentials were induced in EC stellate cells (50 action potentials, black trace), and postsynaptic responses were recorded in dentate granule cells (gray traces, single sweeps; blue trace, averaged response). F, Synaptic failure rate was not significantly different between paired recordings from the lateral and the medial part of the perforant path. Nevertheless, monosynaptic connections between the medial layer 2 stellate cells and dentate granule cells had significantly faster rise times (nEC(lat)-GC = 24 pairs in 9 tissue cultures; nEC(med)-GC = 21 pairs in 10 tissue cultures; Mann–Whitney test, U = 162). G, No differences in uEPSC amplitude or area were found between connected neuronal pairs of the lateral and the medial perforant path (Mann–Whitney test). H, Sample traces for STP experiments at either 20 or 10 Hz presynaptic action potential induction. Blue traces indicate averaged responses. I, Short-term plasticity was assessed by the induction of five action potentials at 20 and 10 Hz frequency in presynaptic stellate cells (20 Hz, nEC(lat)-GC = 24 pairs in 9 tissue cultures; nEC(med)-GC = 21 pairs in 10 tissue cultures; 10 Hz: nEC(lat)-GC = 11 pairs in 4 tissue cultures; nEC(med)-GC = 9 pairs in 4 tissue cultures; normalized to first response/baseline). EC(lat)–GC pairs displayed a synaptic facilitation at 20 Hz, whereas neither facilitation nor depression was seen at 10 Hz presynaptic stimulation. In contrast, EC(med)–GC showed in both 20 Hz and 10 Hz presynaptic stimulation neither synaptic facilitation nor depression in response to the second presynaptic action potential. lat, Lateral; med, medial. Repetitive stimulation of both pathways resulted in progressive synaptic depletion, which seemed more prominent in 20 Hz stimulation. Thus, significant differences between the individual pathways on repetitive presynaptic stimulation at the synaptic level were identified (RM 2-way ANOVA with Sidak's multiple comparisons test). Individual data points are indicated by gray dots. Values represent mean ± SEM (*p < 0.05, **p < 0.01; ns, not significant).
