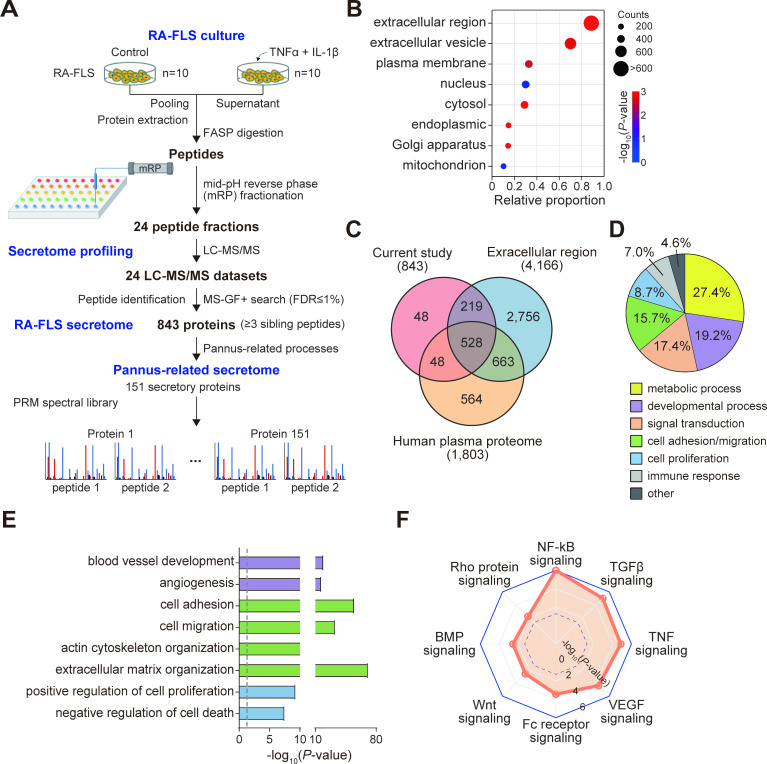
Figure 1.
Characteristics of RA-FLS secretome. (A) Overall scheme illustrating experimental steps for RA-FLS culture, secretome profiling, RA-FLS secretome and pannus-related secretome. For pro-inflammatory stimulation, 10 different RA-FLSs were treated with IL-1β (10 ng/mL, n=5), TNFα (10 ng/mL, n=5) or medium alone (n=10) for 24 hours in DMEM containing 1% insulin-transferrin-selenium used as an alternative of FBS. (B) Relative proportions of RA-FLS secretory proteins localised in the indicated Gene Ontology cellular components. The size and colour of dots represent the number of proteins localised in the cellular components and the enrichment p value from DAVID software, respectively. (C) Relationships among RA-FLS secretome, proteins localised in extracellular region and human plasma proteome. (D) Relative proportions of RA-FLS secretory proteins involved in cellular processes related to the indicated processes. (E) GOBPs significantly enriched in RA-FLS secretory proteins. Enrichment significance (p value) was displayed as −log10(p value). Bar colours represent the corresponding cellular processes in D. (F) Signalling pathways represented by RA-FLS secretory proteins. Enrichment significance (p value) was displayed as −log10(p value). The dotted line indicates the cut-off of p value (0.05). DMEM, dulbecco's modified eagle medium; FASP, filter aided sample preparation; FBS, fetal bovine serum; FLS, fibroblast-like synoviocyte; GOBPs, gene ontology biological processes; IL, interleukin; NF-κB, nuclear factor kappa B; PRM, parallel reaction monitoring; RA, rheumatoid arthritis; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor.