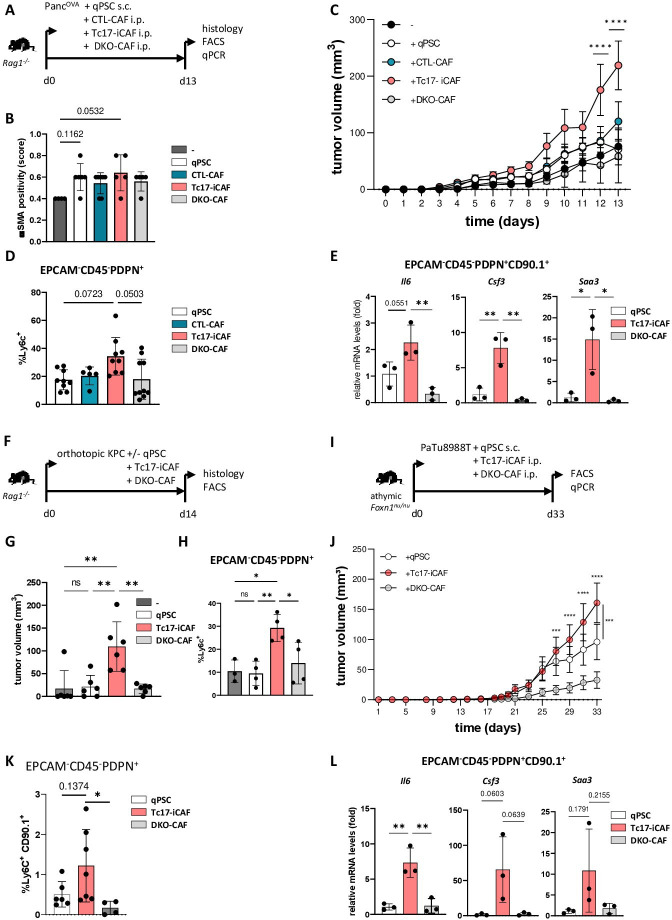
Figure 4.
Tc17-iCAF promote pancreatic tumour growth in vivo. (A) Scheme of the experimental design. 5×105 PancOVA tumour cells ±5×105 in vitro differentiated CD90.1+qPSC, CD90.1+CTL-CAF, CD90.1+Tc17-iCAF or CD90.1+DKO-CAF were subcutaneously co-injected into immunodeficient Rag1-/- mice. Histology and CAF analyses were performed at the indicated end of the experiment. (B) Quantification of αSMA staining in tumour tissue of mice injected with PancOVA cells alone (-) or co-injected with qPSC (qPSC), with CTL-CAF (CTL-CAF), with Tc17-iCAF (Tc17-iCAF) or with DKO-CAF (DKO-CAF), based on previously published scoring,17 (n=4–7). (C) Tumour-growth curve of subcutaneous tumours is shown (tumour volume in MM3; mean±SEM, n=5–7, a representative of two independent experiments each with 5–7 mice). ****p<0.0001 indicates the tumour volume comparisons between mice with Tc17-iCAF vs qPSC injection. (D) FACS analysis of Ly6chigh cell frequency in gated EPCAM-CD45-PDPN+ fibroblasts in subcutaneous tumours (n=5–10). (E) qPCR analysis of the indicated genes expressed by transferred EPCAM-CD45-PDPN+CD90.1+ fibroblasts sorted from subcutaneous tumours (mean±SD, n=3). Fold of mRNA expression is shown, normalised to the qPSC group, which was arbitrarily set to 1, (n=3). (F) Scheme of the experimental design. 2×104 KPC tumour cells±2 × 104 CD90.1+qPSC, CD90.1+Tc17-iCAF or CD90.1+DKO-CAF were orthotopically co-injected into Rag1-/- mice. The tumour volume and CAF phenotype were analysed at the indicated end of the experiment. (G) Tumour volume of orthotopic tumours of mice injected with KPC cells alone (-) or co-injected with qPSC (qPSC), with Tc17-iCAF (Tc17-iCAF) or with DKO-CAF (DKO-CAF) is shown (mean±SD, n=6 mice). (H) FACS analysis of Ly6chigh cell frequency in gated EPCAM-CD45-PDPN+ cells in orthotopic tumours of mice treated as indicated (mean±SD, n=3–4). (I) Scheme of the experimental design. 5×105 PaTu8988T human PDAC cells together with 5×105 in vitro differentiated CD90.1+qPSC or CD90.1+Tc17-iCAF or CD90.1+DKO-CAF or were co-injected subcutaneously into immunodeficient athymic Foxn1nu/nu nude mice. CAF analysis was performed at the indicated end of the experiment. (J) Tumour-growth curve of subcutaneous tumours (MM3) of mice co-injected with PaTU8988T cells and with qPSC (qPSC) or with Tc17-iCAF (Tc17-iCAF) or with DKO-CAF (DKO-CAF) (mean±SEM, n=10 mice). ***p<0.001 and ****p<0.0001 above the PaTu8988T-Tc17-iCAF curve indicate the comparisons between mice with Tc17-iCAF vs DKO-iCAF co-injection. ***p<0.0005 on the right side of the graph indicates the comparison between mice with Tc17-iCAF vs qPSC co-injection. (K) FACS analysis of Ly6chigh cell frequency in gated EPCAM-CD45-PDPN+ fibroblasts in subcutaneous tumours (n=4–6). (L) qPCR analysis of the indicated gene expression by sorted from subcutaneous tumours transferred EPCAM-CD45-PDPN+CD90.1+ fibroblasts (mean±SD, n=3). Fold of mRNA expression is shown, normalised to the qPSC group, which was arbitrarily set to 1 (n=3). (B, D, E, G, H, J, K, L) biological replicates are plotted. In (B, D, K) statistics by Kruskal-Wallis-Test, *p<0.05, ns (non-significant) (C, J) ***p<0.001, ****p<0.0001 determined by two-way ANOVA with Bonferroni post hoc test, (E, G, H, L) *p<0.05, **p<0.01 and p values by one-way ANOVA followed by Tukey’s HSD multiple comparison test. αSMA, a-smooth muscle actin; ANOVA, analysis of variance; HSD, honestly significant difference; iCAF, inflammatory cancer-associated fibroblasts; qPSC, quiescent pancreatic stellate cell.