Figure 4.
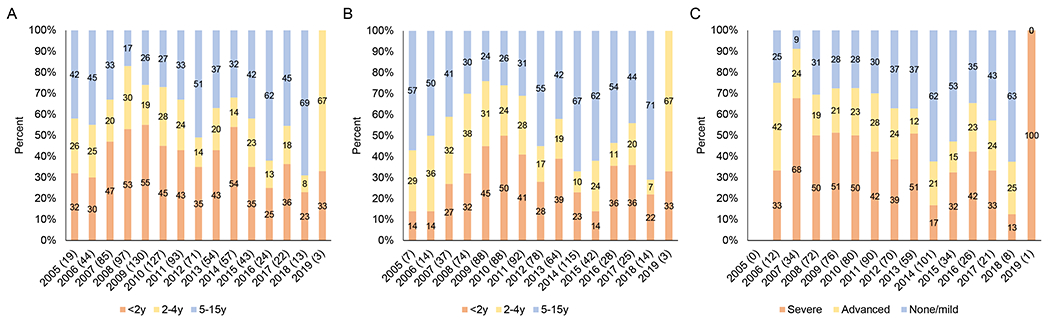
Distribution among study participants of A) age at registration for care at the Macha HIV clinic by year; B) age at treatment initiation by year; C) level of immune suppression at treatment initiation by year
Note: Sample sizes are provided in parentheses after the year. Children who transferred to the Macha HIV clinic already receiving treatment were excluded. Immune suppression defined according to WHO guidelines based on age and CD4+ T-cell percentage or count (0-11 months: none/mild - ≥30%, advanced – 25-29%, severe - <25%; 12-35 months: none/mild - ≥25%, advanced – 20-24%, severe - <20%; 36\59 months: ≥20%, advanced – 15\19%, severe - <15%; ≥5 years: none/mild - ≥350, advanced – 200\349, severe - <200 or <15%).19
