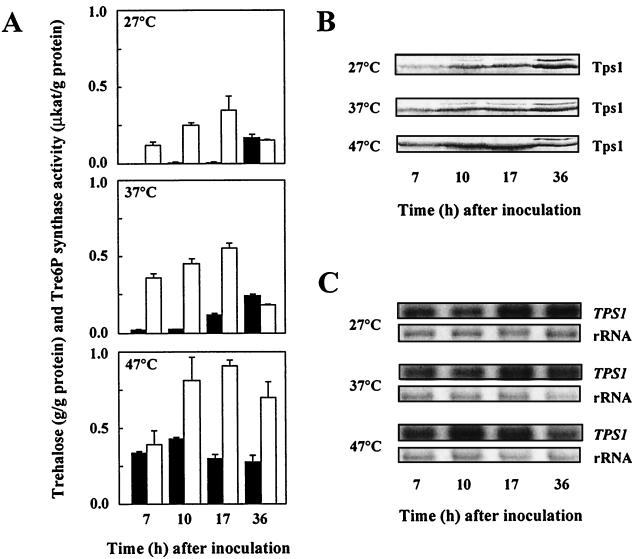
FIG. 1.
Levels of trehalose and Tre6P synthase activity (A), Tps1 protein (B), and TPS1 mRNA (C) of wild-type H. polymorpha cells grown to stationary phase at 27, 37, and 47°C. Cultures were pregrown overnight at the temperatures indicated and diluted (at time zero) with fresh YPD medium to an initial optical density at 600 nm of 0.2. Samples were taken in exponential phase (7 h), late exponential phase (10 h), early stationary phase (17 h), and late stationary phase (36 h). Glucose was consumed after 12 h (at 27°C), 11 h (at 37°C), or 12 h (at 47°C). (A) Shaded bars denote trehalose levels (determined as described in reference 7), and open bars denote Tre6P synthase activities (assayed as described in references 6 and 7). Error bars indicate standard deviations. (B) Immunoblot analysis of the Tps1 protein levels by using polyclonal rabbit antibodies raised against a peptide (Gly-Val-Asp-Arg-Leu-Asp-Tyr-Ile-Lys-Gly-Val-Pro-Gln-Lys) corresponding to a highly conserved amino acid sequence in Tps1 proteins of various fungi. Several proteins cross-reacted weakly with these antibodies, and the identification of the correct band was, therefore, verified in each case by the use of an extract from an H. polymorpha Δtps1 strain (data not shown). (C) Total RNAs were extracted (14), and equal amounts (10 μg) were probed with an internal 650-bp fragment of the H. polymorpha TPS1 gene after electrophoresis and blotting. The application and transfer of equal amounts of RNA were verified by ethidium bromide staining.