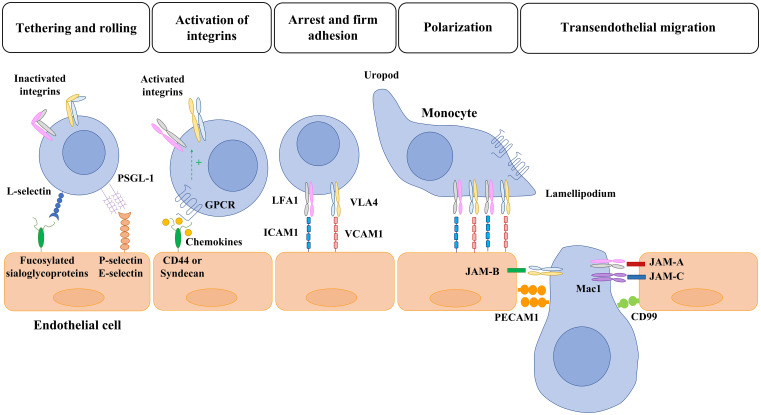
Figure 1.
Overview of monocyte-endothelial cell interaction and transmigration. Monocyte migration to inflammatory sites is a multistep process with many molecules involved. First, selectins mediate the initial tethering and rolling of monocytes along the cytokine-activated endothelial cells. Then, the monocyte interacts with chemokines that are bound to transmembrane heparan sulphate proteoglycans (CD44 and sydecan) expressed in the endothelium. The activation of GPCR leads to the activation of integrins and the consequent monocyte arrest mediated by the interaction of LFA1 and VLA4 with ICAM1 and VCAM, respectively. Once a monocyte establishes firm adhesion to the vascular endothelium, it undergoes a morphological change known as polarization, in which chemokine receptors and activated integrins redistribute to the leading edge. After polarization, monocytes migrate toward interendothelial junctions and then transmigrate into the underlying tissues. The members of the JAM family expressed by endothelial cells (JAM-A, JAM-B, JAM-C) interact with the activated integrins of monocytes (LFA1, VLA4, Mac1) and allow the transmigration through tight junctions. Lastly, PECAM-1 (CD31) and CD99 hemophilic engagement and endothelial retraction lead to monocyte extravasation. PSGL: P-selectin glycoprotein ligand-1; GPCR: G-protein-coupled receptors; LFA1: lymphocyte function-associated antigen 1; VLA4: very late antigen 4; ICAM1: intercellular adhesion molecule 1 (ICAM1/CD54); VCAM: vascular cell-adhesion molecule 1; Mac1: macrophage receptor 1; PECAM: platelet/endothelial cell-adhesion molecule 1.