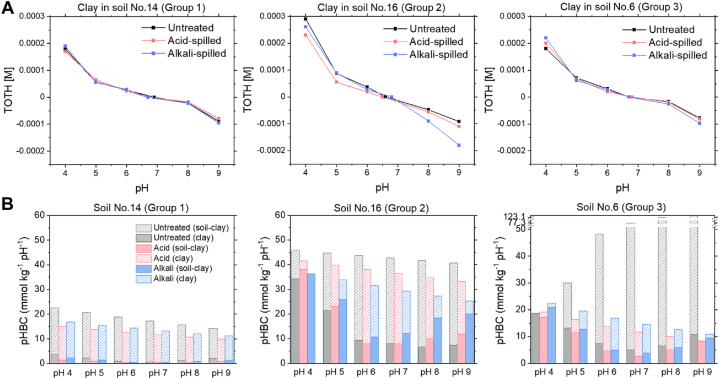
Fig. 3.
(A) Titration curves of clay minerals in untreated, acid-spilled, and alkali-spilled soils (Nos. 14, 16, and 6 for groups 1, 2, and 3, respectively) spills under 0.03 M of NaNO3. The Y-axis is the molar concentration of total proton added to the solution. (B) Contribution of clay minerals and the other components to pH buffering capacity soils (Nos. 14, 16, and 6 for group 1, 2, and 3, respectively) at different pH values. Filled bars represent pH buffering capacity of clay minerals, whereas cross-hatched bars are obtained by subtracting the pH buffering capacity of clay minerals from that of the soil, which is mainly attributed to organic matter.