Fig. 3.
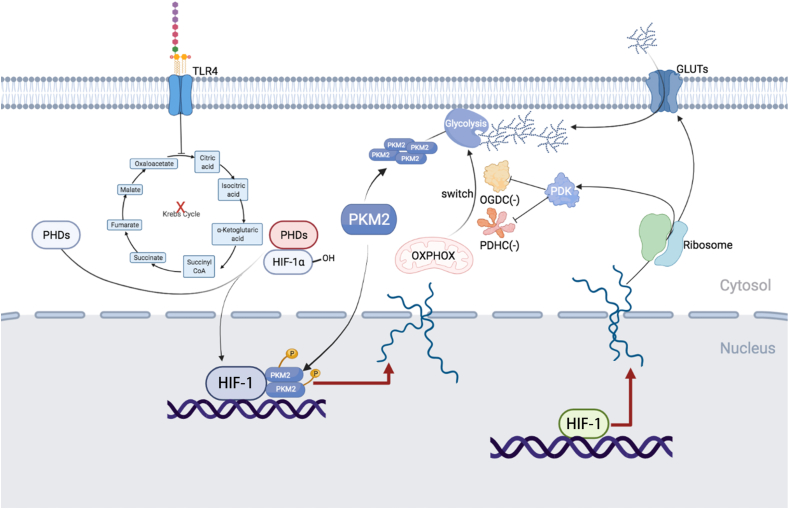
HIF-1α participates in glucose metabolic reprogramming. HIF-1α induces glycolytic gene expression to promote glucose uptake, glycolysis, and tricarboxylic acid cycle (TCA cycle), thereby enhancing HIF-1α stability. Pyruvate dehydrogenase kinase isozyme 1 (PDK1) inhibits pyruvate dehydrogenase complex (PDHC) and oxoglutarate dehydrogenase complex (OGDC), thereby promoting switching from mitochondrial oxidative phosphorylation to aerobic glycolysis. Lipopolysaccharide (LPS), an inflammation inducer, inhibits the TCA cycle and induces succinic acid, fumaric acid, citric acid, and itaconic acid accumulation, resulting in stabilization of HIF-1α. The tetramer pyruvate kinase M2 (PKM2) is primarily cytoplasmic and exhibits high pyruvate kinase activity, which can catalyze phosphoenolpyruvate to generate pyruvate. Dimeric PKM2 can translocate into the nucleus with high protein kinase activity, acting as a transcription factor to regulate downstream transcription. The toll-like receptor 4 (TLR4) signaling pathway stimuli, such as LPS, promote phosphorylation of PKM2 to maintain the monomer/dimer state. PKM2 monomer or dimer can form a complex with HIF-1α in the nucleus and significantly increase the transcriptional activity of HIF-1α.