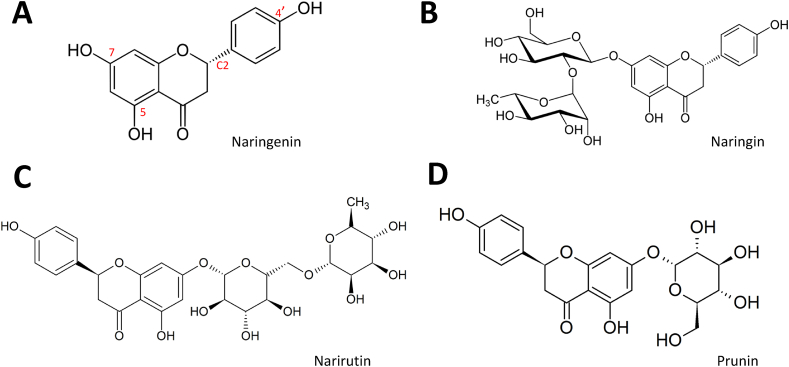
Fig. 3.
Structure of Naringenin and its analogs. (A) Naringenin indicates free hydroxyl groups at the 5, 7, and 4′positions as well as a chiral center at C2. The substitution of OH at position 7 for 2-O-α l-rhamnosyl-D-glucoside gives Naringin (B) and for 6-O-α l-rhamnosyl-D-glucoside forms Narirutin (C). Prunin or Floribundoside is produced by substitutions at positions 5 and 7 of the Naringenin structure with glucose (D).