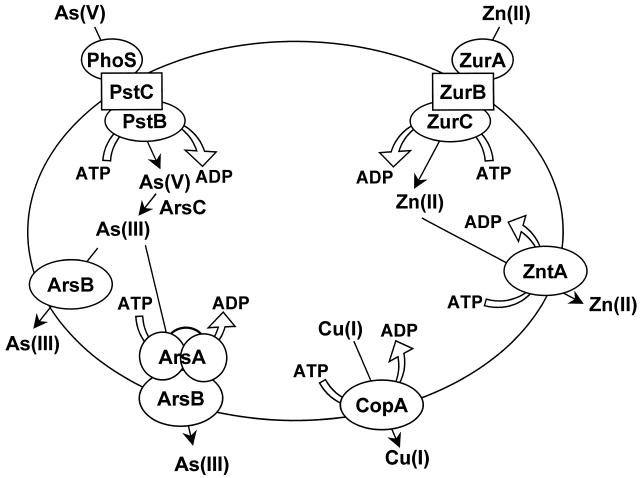
FIG. 1.
Soft metal transport ATPases. In E. coli there are primary pumps for the uptake and efflux of transition metals, heavy metals, and metalloids. (Left) Arsenate is accumulated by phosphate transporters such the Pst pump (51), a member of the ABC superfamily of transport ATPases. Arsenate is reduced to arsenite via the ArsC arsenate reductase. Arsenite is then extruded from cells by the Ars extrusion system (44). In cells expressing the chromosomal arsRBC operon, ArsB is a secondary arsenite carrier protein coupled to the membrane potential that confers low-level resistance to metalloid salts. In contrast, in cells expressing a plasmid-encoded arsRDABC operon, the ArsAB complex is an As(III)/Sb(III)-translocating ATPase that confers high level resistance. (Middle) The CopA P-type ATPase confers resistance to copper ion and probably transports the monovalent cation. (Right) ZnuABC is an ABC transport ATPase that catalyzes uptake of Zn(II) (37), and ZntA is a Zn(II)-translocating P-type ATPase (2, 42). It is hypothesized that these two pumps are a homeostatic mechanism that controls the intracellular concentration of Zn(II). In addition, ZntA confers resistance to Pb(II), Cd(II), and excess Zn(II).