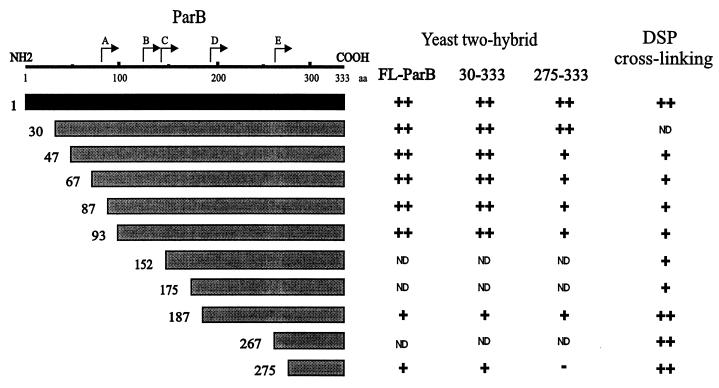
FIG. 6.
Summary of dimerization assays with C-terminal fragments of ParB. In yeast two-hybrid analysis, the ParB fragments shown in the diagram were fused to the GAL4 activation domain and were tested against FL-ParB, 30-333 ParB, and 275-333 ParB (in the columns) that were fused to the GAL4 DNA binding domain. For the cross-linking experiments, ParB fragments fused to a polyhistidine tag (Table 2) were purified and examined in vitro (Materials and Methods). The results from the yeast two-hybrid experiments were categorized as follows: −, no color development on filter tests or no growth on plates without histidine; +, moderate color development and moderate growth in the absence of histidine; ++, dark blue color and good growth in the absence of histidine. ND, not determined. The DSP cross-linking results were similarly categorized: −, no cross-linking; +, some cross-linking activity; ++, strong cross-linking, often to completion. Neither set of categories is intended to imply relative strengths of the interactions, which are presumably dependent on the assay. The N termini of the tryptic proteolytic fragments are indicated above the schematic of ParB.