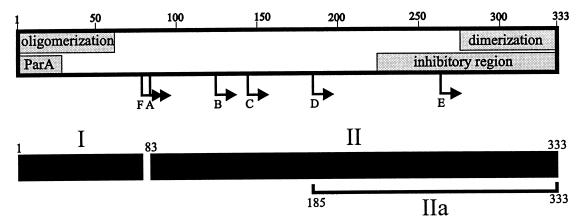
FIG. 9.
Model of ParB’s functional and structural domains. The shaded boxes represent regions of the protein involved in ParB-ParB and ParA-ParB interactions. Arrows indicate the N termini of the proteolytic fragments (A to F) identified in this study. We propose that the C-terminal self-association domain is required for ParB dimerization and the N-terminal self-association domain mediates oligomerization. The inhibitory region affects the self-association of ParB that is mediated by the N-terminal oligomerization domain, as measured by cross-linking assays. The ParA box indicates a region of ParB that is necessary for interactions with ParA. The lower black boxes predict the general structural domains of ParB, from proteolytic assays. Region I represents the protease-accessible N-terminal region, which may mean that it is less structured in solution. Region II is more stable and more protease resistant. Region IIa represents the smallest highly protease resistant region of ParB.