Figure 2: Regulating classical cadherin adhesion.
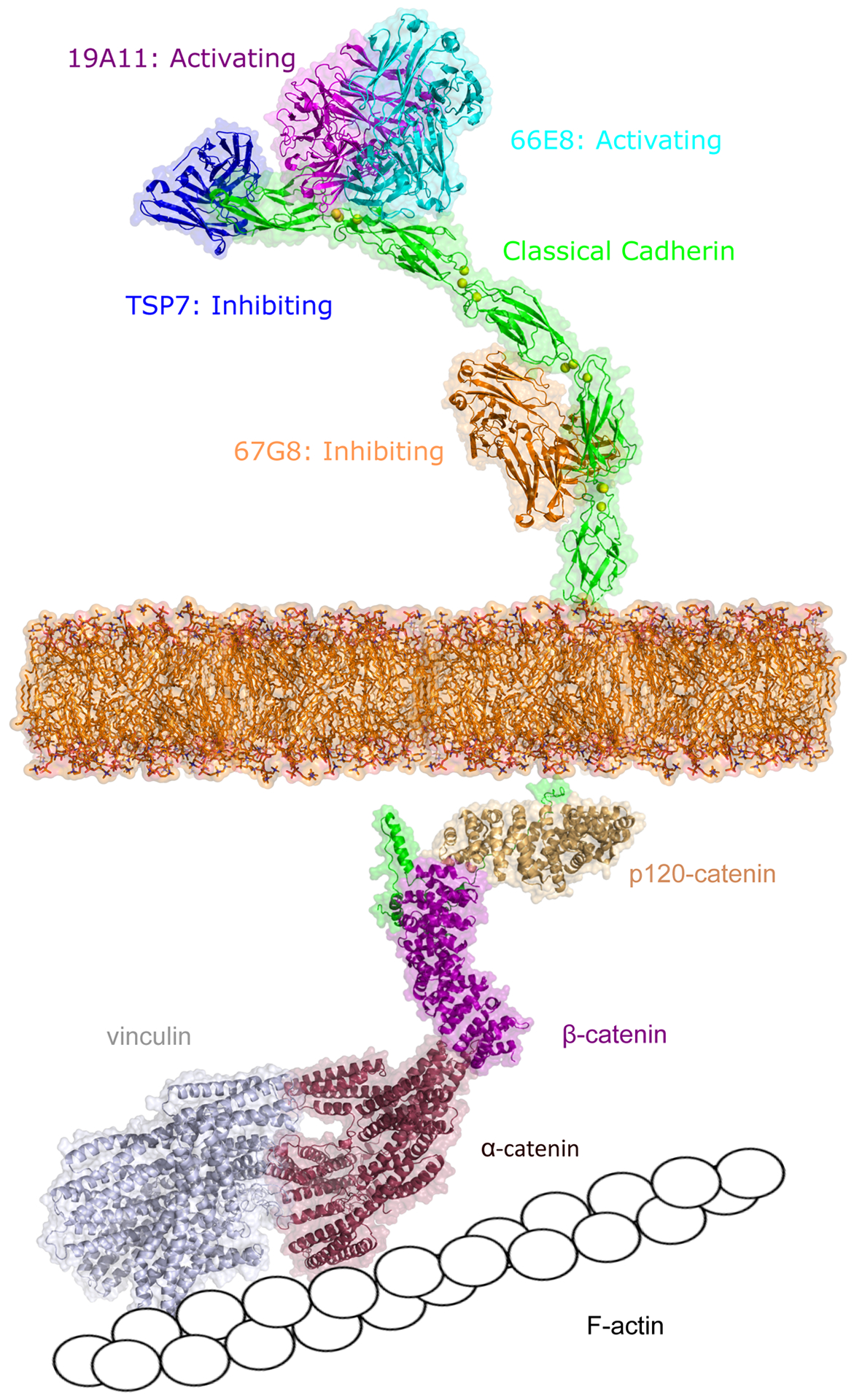
Cadherin adhesion can be regulating either via extracellular binding with antibodies or from the inside-out by cytoplasmic proteins. Activating antibodies 19A11 (magenta, PDB: 6CXY) and 66E8 (cyan, PDB:6VEL) recognize E-cadherin EC1 or EC2 domain (green, PDB: 3Q2V) near the cadherin trans binding sites. Antibody TSP7 (blue, PDB: 5JYL) inhibits P-cadherin adhesion by binding on the EC1 domain while 67G8 (orange) inhibits E-cadherin adhesion by binding on EC5 domain. The intracellular domain of cadherin (green) associates with various signaling molecules, including β-catenin (pink, PDB: 3L6X), p120-catenin (light yellow, PDB: 4R10), α-catenin (red), and vinculin (grey), which eventually link the cadherin cytoplasmic region to F-actin. The structures of α-catenin, vinculin, and E-cadherin intracellular domain were predicted using Alphafold (94). Unless apparent from the crystal structure, all interactions were predicted using Alphafold.
