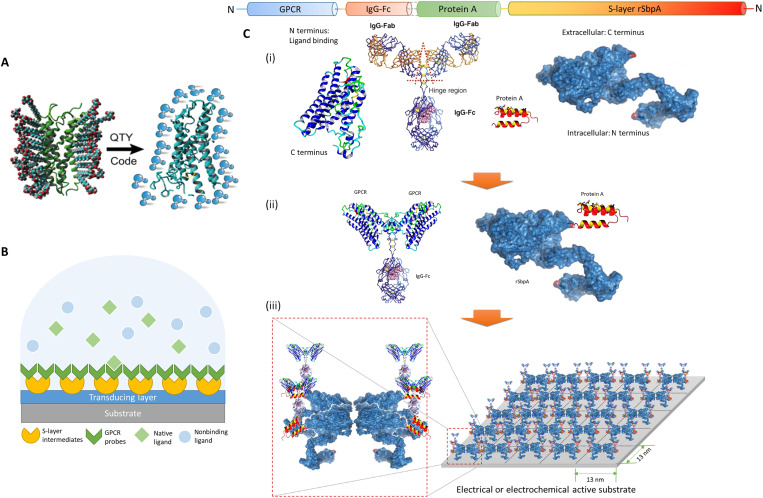
Fig. 1. Schematic illustration of the architecture.
(A) The QTY code transforms hydrophobic membrane receptors to water-soluble functional equivalents that do not require detergents to stabilize in aqueous solutions. (B) S-layer proteins and solubilized receptors are sequentially coated onto designated sensing devices. Target analytes are specifically recognized in aqueous media, while the detection signal is transduced for electrical responses. GPCR, G protein–coupled receptor. (C) Sequence layout of the dual-monolayer construct. (i) The four elements of the construct: redesigned water-soluble membrane receptor, heavy chain Fc region of immunoglobulin G (IgG) protein, Fc-binding fragment of protein A, and two-dimensional (2D) crystal-forming S-layer protein. (ii) Solubilized receptors are fused to IgG-Fc to serve as the biospecific probes, while S-layer proteins are fused with protein A fragments to connect probes to the device. (iii) Sequentially, S-layer/protein A fusion proteins reproduce its native nanoscale 2D crystalline lattice on the substrate, after which the receptor probes with Fc fusions were applied to bind the intermediate layer in high affinity and capacity. The specific construct presented here (rSbpA-ZZ/CXCR4QTY-Fc) has p4 symmetry, a lattice parameter of ~13 nm2, and a receptor density in the order of ~1012 U/cm2.